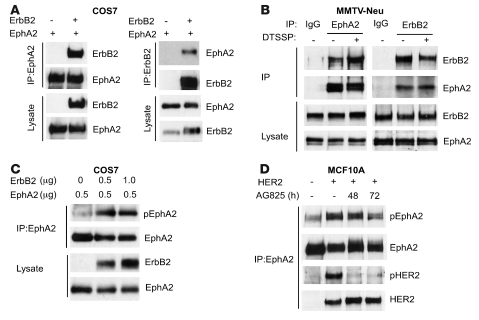
Figure 6. EphA2 physically and functionally interacts with ErbB2.
(A) COS7 cells were transfected with plasmids for expression of EphA2 or/and ErbB2. EphA2 or ErbB2 was immunoprecipitated from cell lysates, and products were analyzed for ErbB2 or/and EphA2. Coexpression of EphA2 and ErbB2 was sufficient to permit coimmunoprecipitation. (B) Endogenous ErbB2 and EphA2 were coimmunoprecipitated with anti-EphA2 or anti-ErbB2 antibodies, respectively, in EphA2+/+ MMTV-Neu tumor cells that were untreated or treated with the chemical crosslinker DTSSP. The interaction detected was specific: EphA2 and ErbB2 were not immunoprecipitated by control IgG. Uniform input was validated by probing lysates for expression of EphA2 and ErbB2. (C) COS7 cells were transfected with plasmids for expression of EphA2 or ErbB2. EphA2 was immunoprecipitated from cell lysates, and products were analyzed for EphA2 expression and tyrosine phosphorylation. Coexpression of ErbB2 and EphA2 was sufficient to induce phosphorylation of EphA2 in COS7 cells in the absence of ephrin ligand stimulation. (D) Interaction between EphA2 and HER2 in MCF10A cells overexpressing HER2 was observed, as EphA2 and HER2 were coimmunoprecipitated with anti-EphA2 antibodies in HER2-overexpressing cells, but not in parental MCF10A cells. Elevated EphA2 phosphorylation was observed in MCF10A cells overexpressing HER2 relative to parental MCF10A cells, and treatment with the ErbB2 kinase inhibitor AG825 reduced EphA2 phosphorylation as well as ErbB2 phosphorylation in MCF10A cells overexpressing HER2.