Abstract
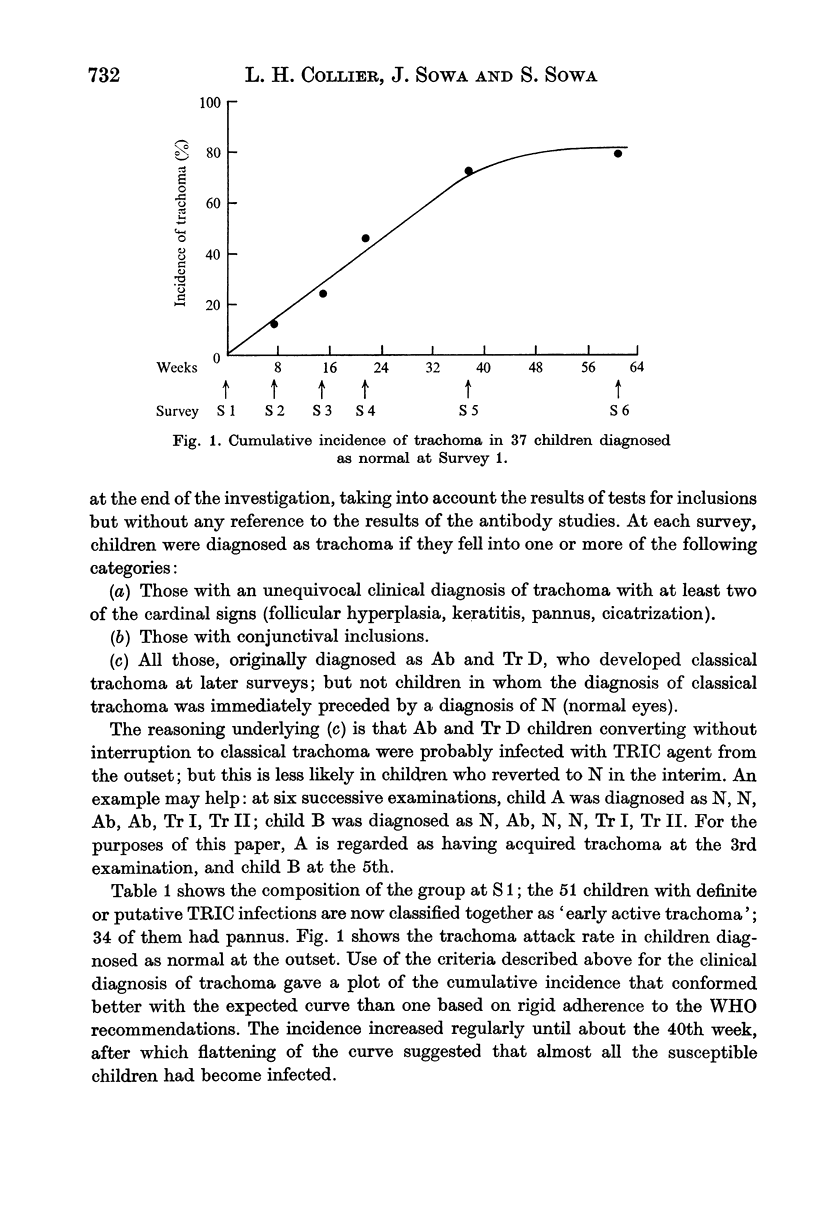
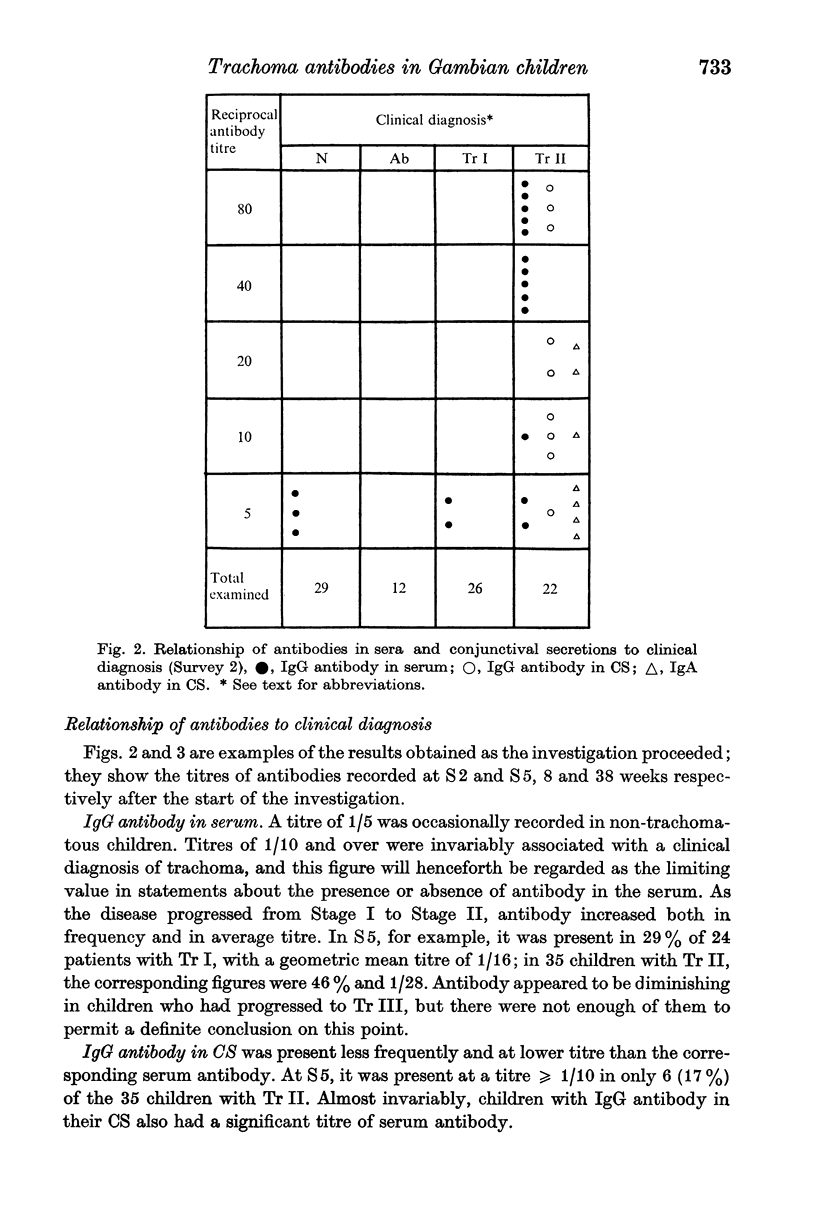
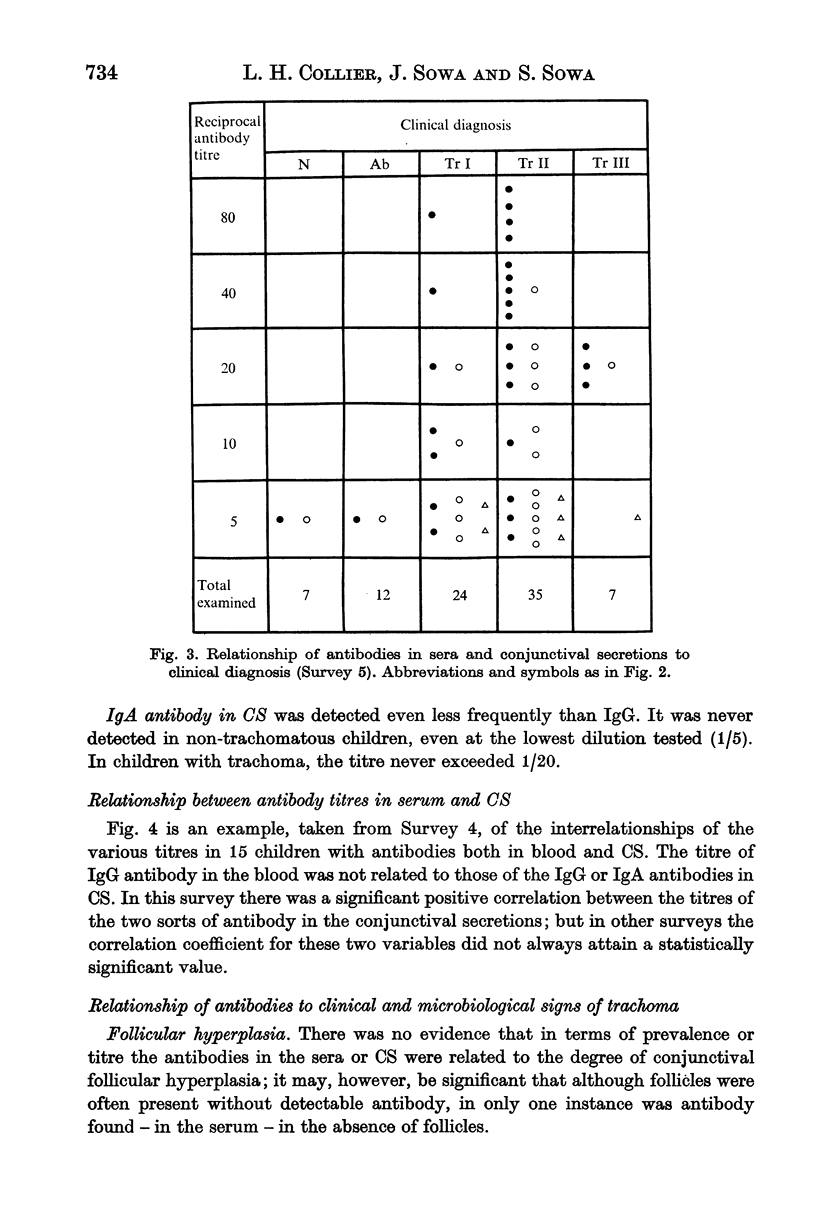
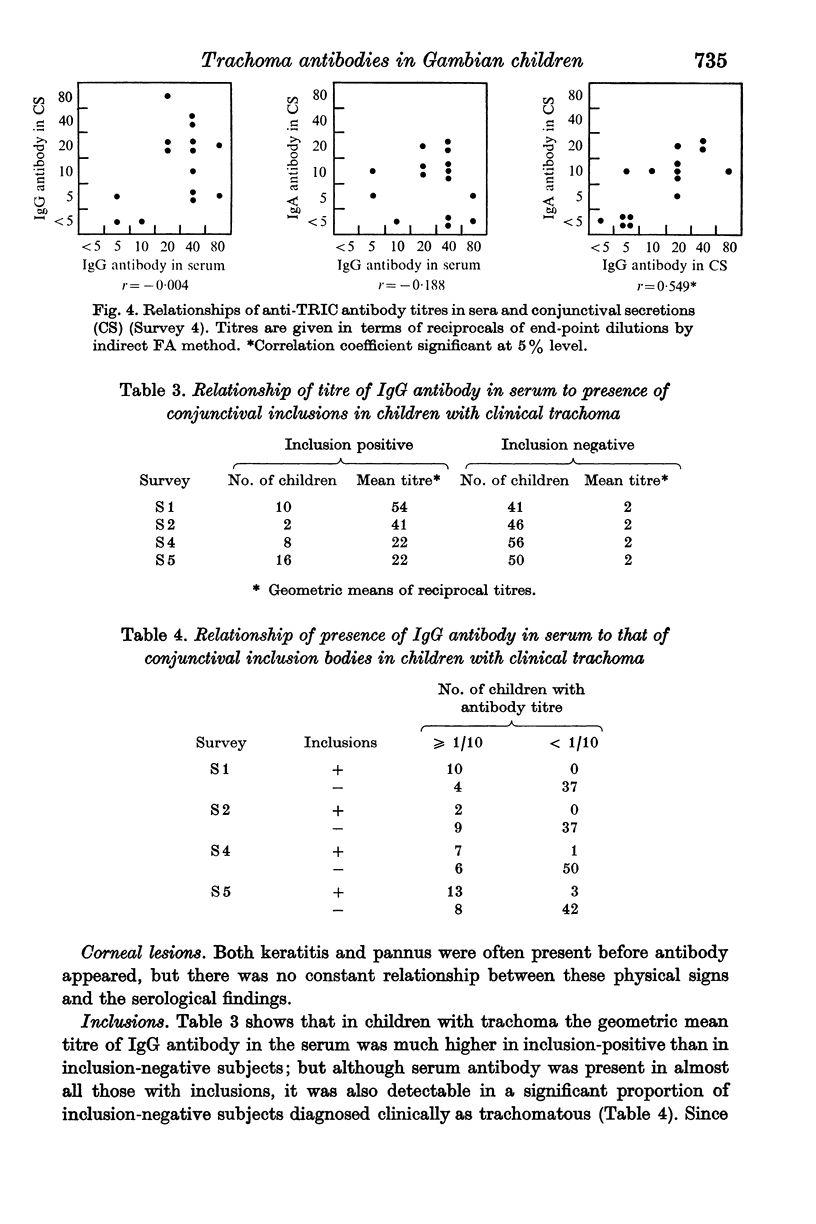
Ninety-nine young Gambian children were studied for 61 weeks. About half of them had trachoma at the outset, and 80% of the remainder acquired the disease while under observation. IgG trachoma antibody in the serum and IgG and IgA antibodies in the conjunctival secretions (CS) were titrated by an indirect immunofluorescence method. In serum samples obtained in capillary tubes the mean titre was slightly higher than in samples collected on filter paper. Serum antibody at titres ≥ 1/10 was invariably associated with a clinical diagnosis of trachoma; it increased both in frequency and titre as the disease progressed, and was present in about half of those with Tr II. In CS, IgG antibody was present less often and at lower titres than in serum, and IgA antibody was detected even less frequently. There was some evidence of correlation between the titres of IgG and IgA antibodies in CS, but none for a relationship between the titres of the antibodies in serum and those in CS. Antibodies were almost never present in the absence of conjunctival follicles, but their titres were unrelated to the degree of follicular hyperplasia; there was no obvious relationship between the serological findings and corneal lesions. In children diagnosed clinically as trachoma, serum antibody was present in almost all those with conjunctival inclusions, and in a proportion of inclusion-negative subjects; the mean titre was much higher in the inclusion-positive group.
These findings do not settle whether CS antibodies are made locally, or are derived partly or wholly from the blood. They suggest that the indirect immunofluorescence test may be a useful diagnostic aid in trachoma, particularly in view of the rarity of false positive reactions; but there is at present little to choose between it and complement-fixation tests in terms of sensitivity.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander E. R., Wang S. P., Grayston J. T. Further classification of TRIC agents from ocular trachoma and other sources by the mouse toxicity prevention test. Am J Ophthalmol. 1967 May;63(5 Suppl):1469–1478. doi: 10.1016/0002-9394(67)94133-5. [DOI] [PubMed] [Google Scholar]
- BELL S. D., Jr, SNYDER J. C., MURRAY E. S. Immunization of mice against toxic doses of homologous elementary bodies of trachoma. Science. 1959 Sep 11;130(3376):626–627. doi: 10.1126/science.130.3376.626. [DOI] [PubMed] [Google Scholar]
- Bernkopf H., Orfila J., Maythar B. Fluorescent antibodies in the fluid of the conjunctival sac of trachoma patients. Nature. 1966 Feb 12;209(5024):725–726. doi: 10.1038/209725a0. [DOI] [PubMed] [Google Scholar]
- Brighton W. D. Assay of materials used in immunofluorescence techniques. J Clin Pathol. 1966 Sep;19(5):456–460. doi: 10.1136/jcp.19.5.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighton W. D., Lampard J. E. Impurities in conjugated globulins for immunofluorescence techniques. Immunology. 1970 Feb;18(2):319–321. [PMC free article] [PubMed] [Google Scholar]
- Collier L. H. The immunopathology of trachoma: some facts and fancies. Arch Gesamte Virusforsch. 1967;22(1):280–293. doi: 10.1007/BF01240523. [DOI] [PubMed] [Google Scholar]
- Collier L. H. The use of graded density filters made by photography in fluorescence microscopy. J Pathol Bacteriol. 1968 Jul;96(1):238–242. doi: 10.1002/path.1700960130. [DOI] [PubMed] [Google Scholar]
- Gerloff R. K., Watson R. O. The radioisotope precipitation test for psittacosis group antibody. Am J Ophthalmol. 1967 May;63(5 Suppl):1492–1498. doi: 10.1016/0002-9394(67)94137-2. [DOI] [PubMed] [Google Scholar]
- Hanna L., Jawetz E., Nabli B., Hoshiwara I., Ostler B., Dawson C. Titration and typing of serum antibodies in TRIC infections by immunofluorescence. J Immunol. 1972 Jan;108(1):102–107. [PubMed] [Google Scholar]
- McComb D. E., Nichols R. L. Antibodies to trachoma in eye secretions of Saudi Arab children. Am J Epidemiol. 1969 Oct;90(4):278–284. doi: 10.1093/oxfordjournals.aje.a121071. [DOI] [PubMed] [Google Scholar]
- REEVE P., TAVERNE J. Observatios on the growth of trachoma and inclusion blennorrhoea viruses in embryonate eggs. J Hyg (Lond) 1963 Mar;61:67–75. doi: 10.1017/s0022172400020751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa J., Collier L. H., Sowa S. A comparison of the iodine and fluorescent antibody methods for staining trachoma inclusions in the conjunctiva. J Hyg (Lond) 1971 Dec;69(4):693–708. doi: 10.1017/s0022172400021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa S., Sowa J., Collier L. H., Blyth W. A. Trachoma vaccine field trials in The Gambia. J Hyg (Lond) 1969 Dec;67(4):699–717. doi: 10.1017/s0022172400042157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarizzo M. L., Nabli B., Labonne J. Studies on trachoma. 11. Evaluation of laboratory diagnostic methods under field conditions. Bull World Health Organ. 1968;38(6):897–905. [PMC free article] [PubMed] [Google Scholar]


