Abstract
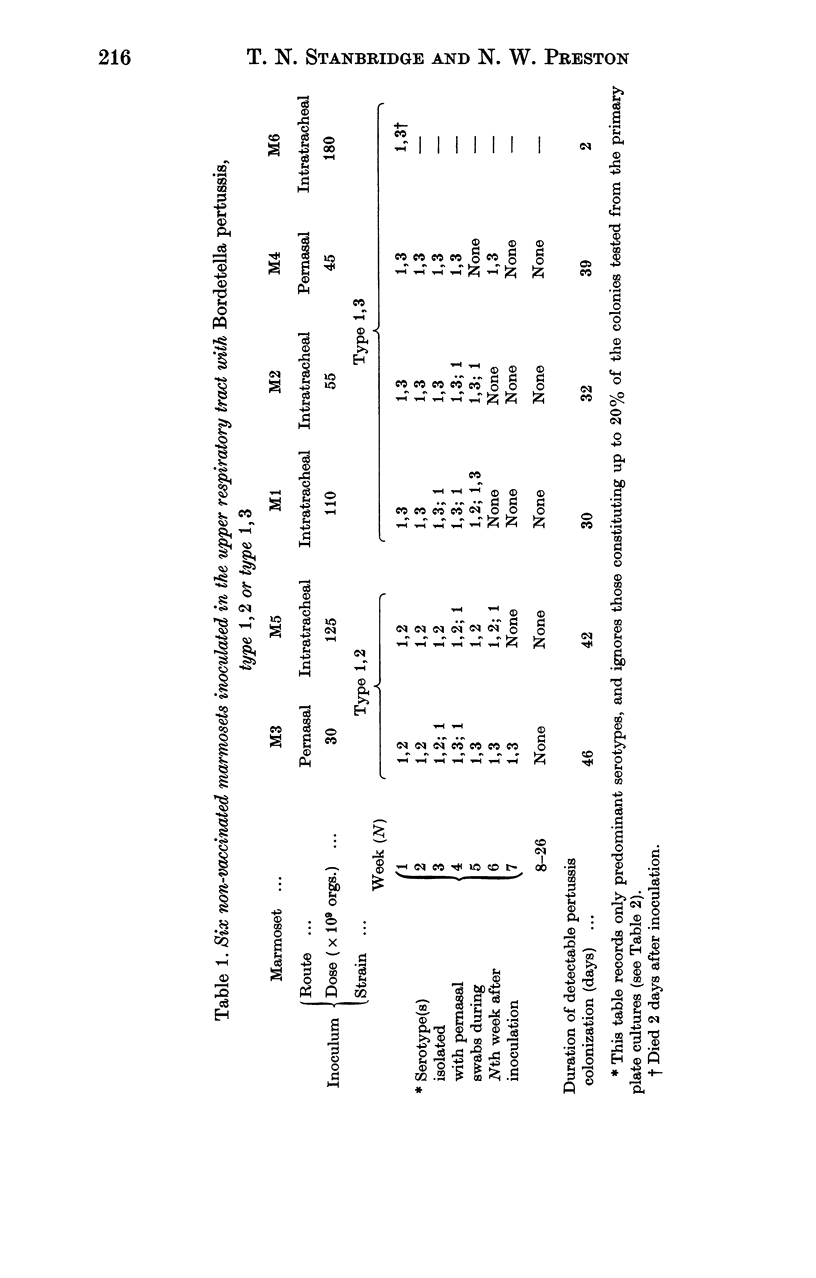
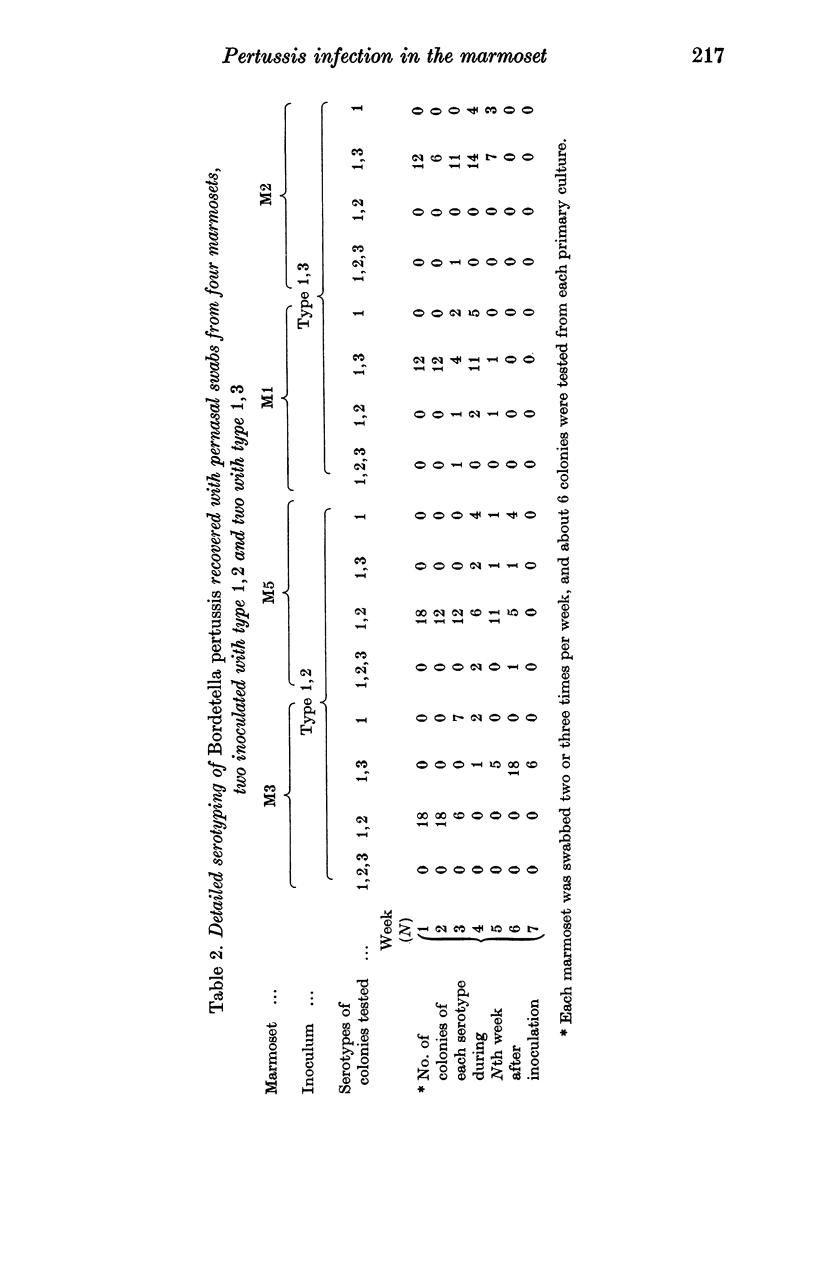
Although we have failed to produce either paroxysmal cough or vomiting in rhesus monkeys, cynomolgus monkeys and marmosets, we have found in marmosets several features of pertussis infection similar to those seen in children with whooping cough: catarrh, persistence of colonization of the naso-pharynx with Bordetella pertussis for 4-11 weeks, change of serotype during colonization and inability of type 1 organisms to establish themselves as the predominant serotype.
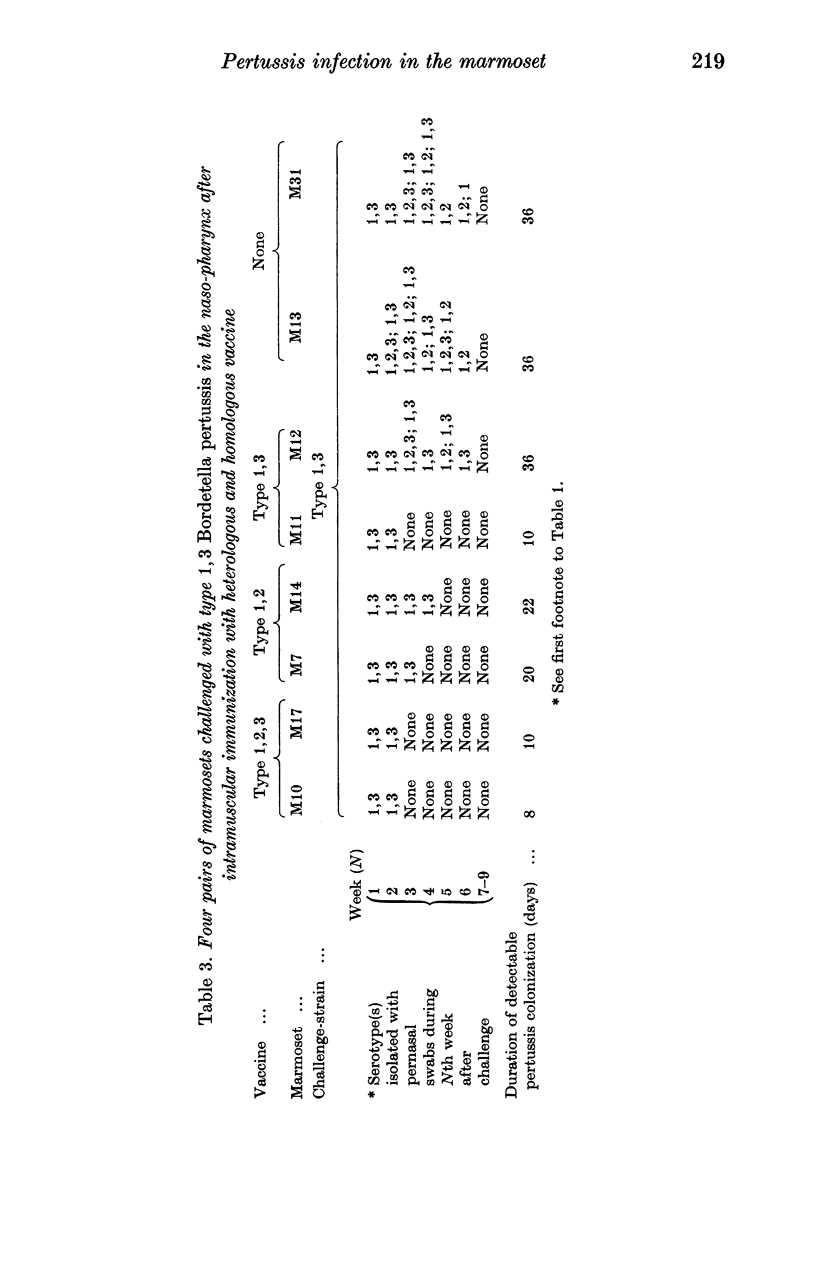
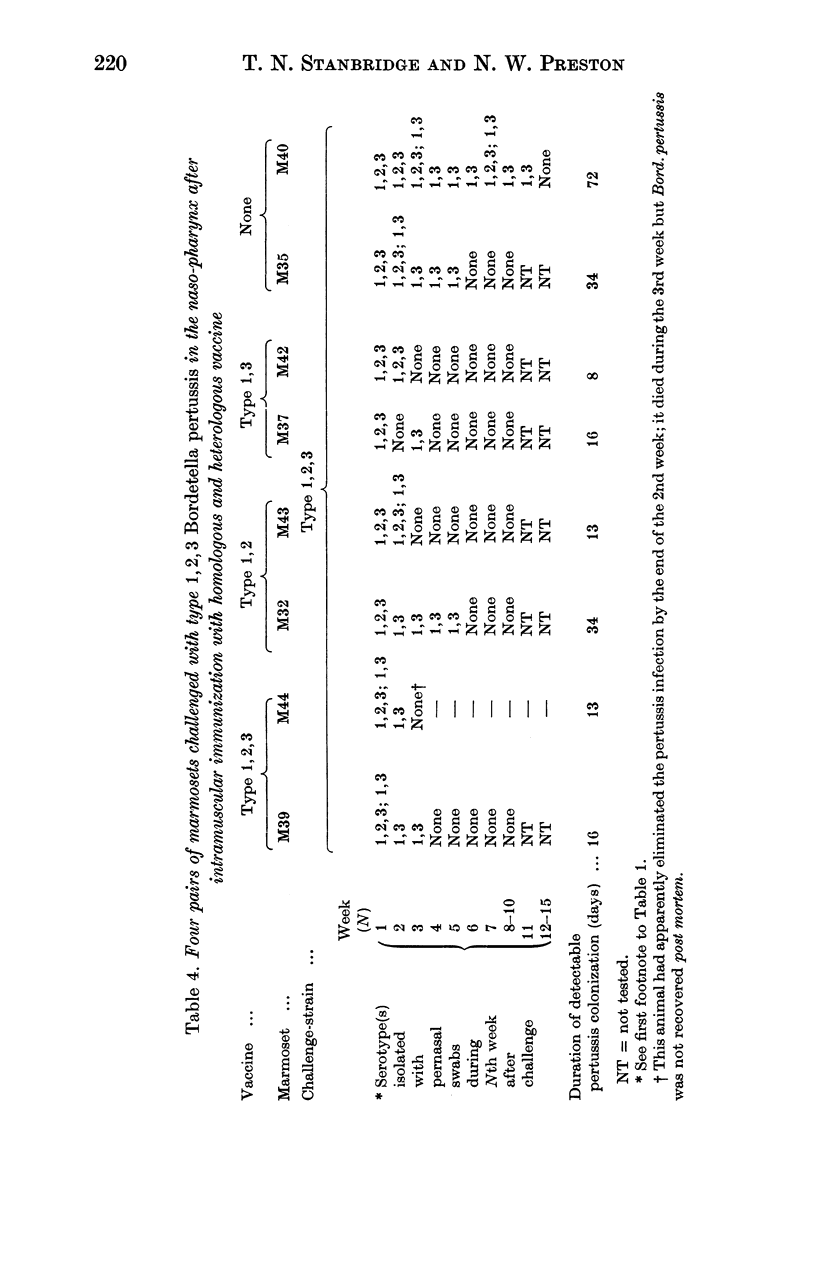
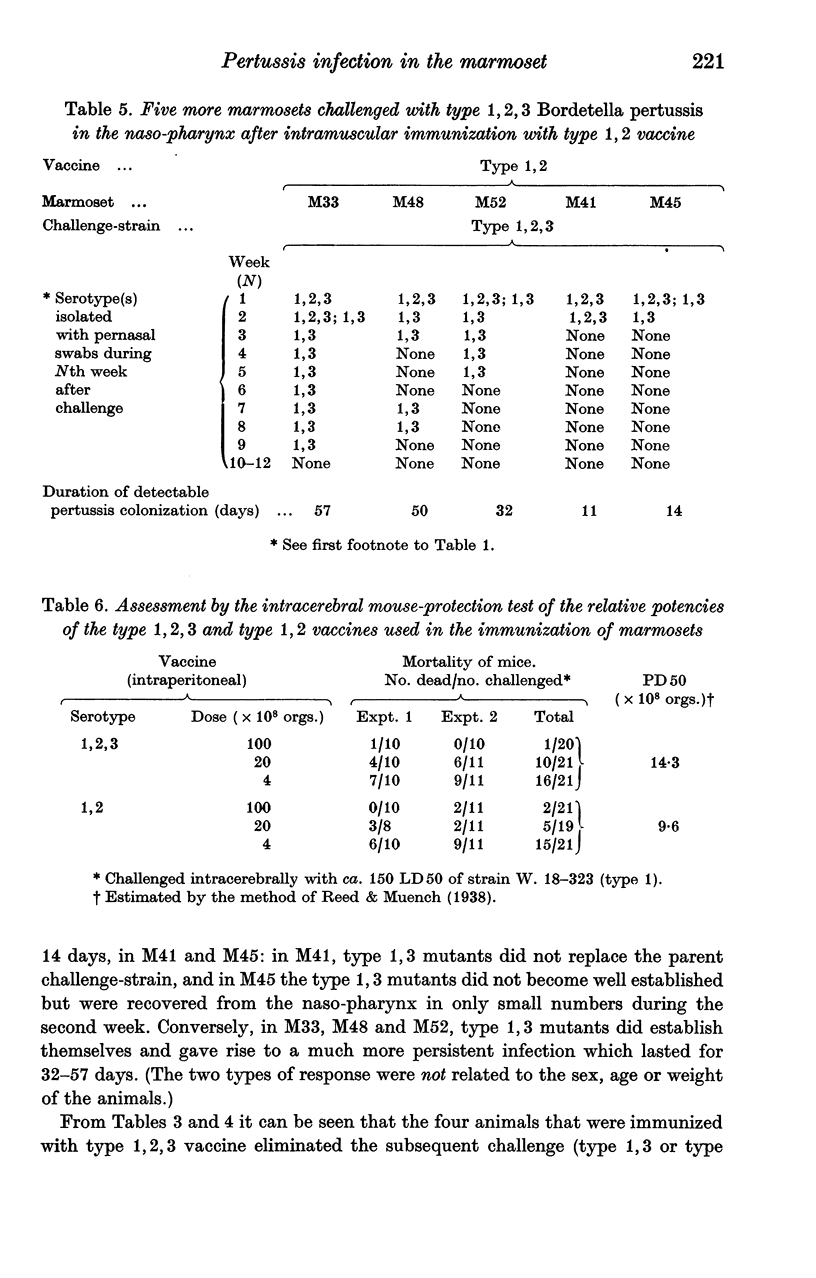
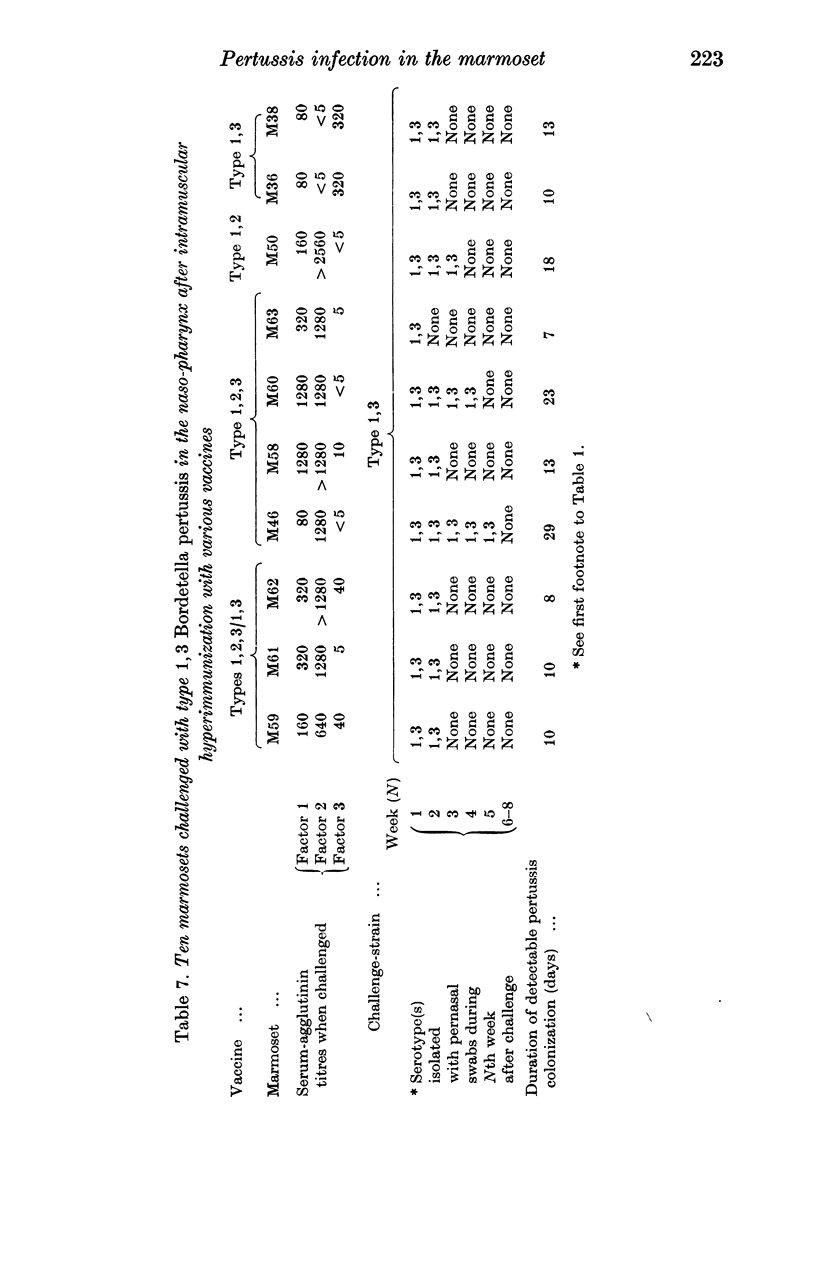
As in children, we have found that intramuscular vaccine of type 1,2,3 was more effective than type 1,2 in preventing persistent infection with the currently prevalent serotypes 1,2,3 and 1,3. A mixed vaccine (1,2,3 and 1,3) seemed to produce agglutinin 3 in the serum more consistently than a pure type 1,2,3 vaccine. The duration of colonization, after naso-pharyngeal challenge, was greatly reduced in animals with agglutinin 3.
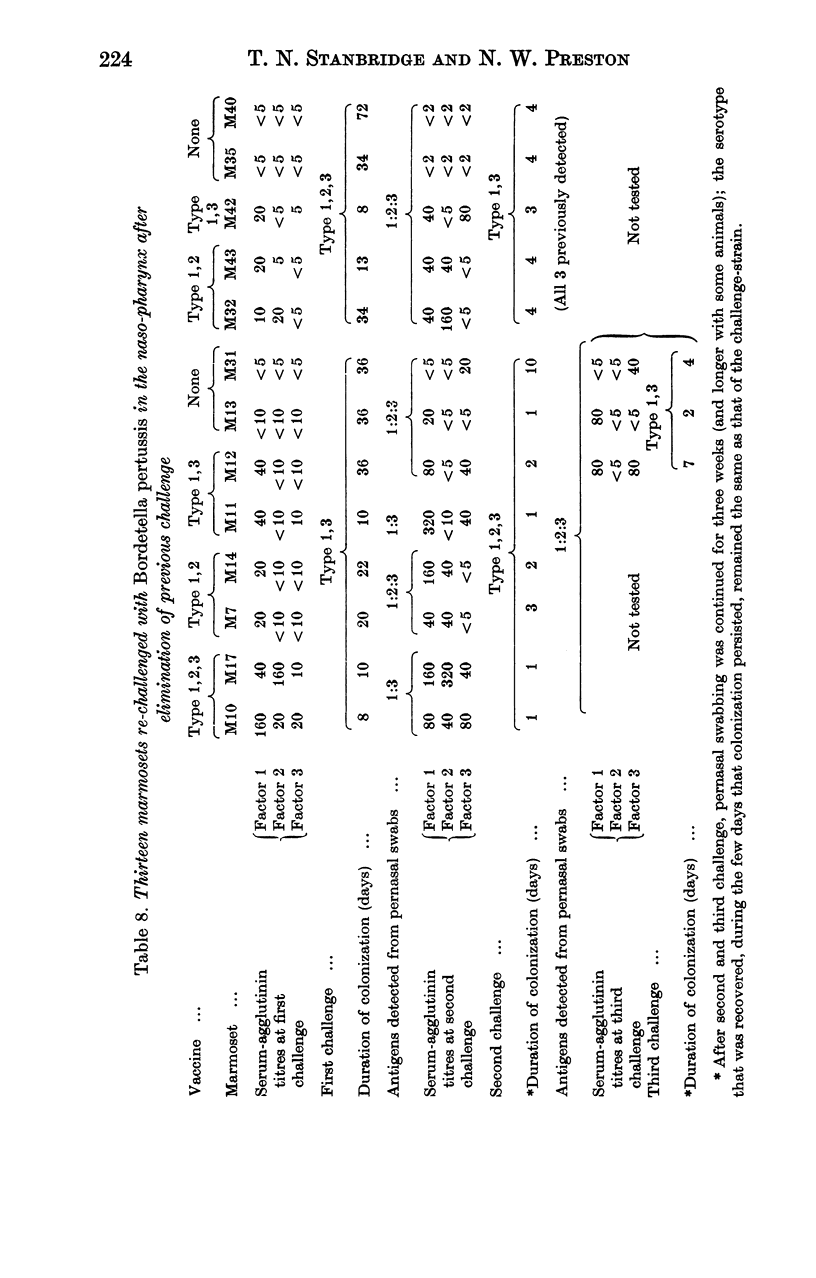
Local immunity, resulting from previous infection, was even more effective than a good vaccine in preventing subsequent persistent colonization. Marmosets may be useful in studying the possible development of aerosol pertussis vaccine for human use.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN E. K., BENTZON M. W. The failure to show correlation between type-specificity and protection in experimental pertussis in mice. Acta Pathol Microbiol Scand. 1958;43(1):106–112. doi: 10.1111/j.1699-0463.1958.tb04877.x. [DOI] [PubMed] [Google Scholar]
- ANDERSEN E. K. Serological studies on H. pertussis H. parapertussis and H. bronchisepticus. Acta Pathol Microbiol Scand. 1953;33(2):202–224. doi: 10.1111/j.1699-0463.1953.tb01512.x. [DOI] [PubMed] [Google Scholar]
- Abbott J. D., Preston N. W., Mackay R. I. Agglutinin response to pertussis vaccination in the child. Br Med J. 1971 Jan 9;1(5740):86–88. doi: 10.1136/bmj.1.5740.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. M., Wheeler M. W. Pertussis Vaccine Prepared with Phase-I Cultures Grown in Fluid Medium. Am J Public Health Nations Health. 1946 Apr;36(4):371–376. [PMC free article] [PubMed] [Google Scholar]
- EVANS D. G., PERKINS F. T. Interference immunity produced by pertussis vaccine to pertussis infection in mice. Br J Exp Pathol. 1954 Dec;35(6):603–608. [PMC free article] [PubMed] [Google Scholar]
- HUANG C. C., CHEN P. M., KUO J. K., CHIU W. H., LIN S. T., LIN H. S., LIN Y. C. Experimental Whooping cough. N Engl J Med. 1962 Jan 18;266:105–111. doi: 10.1056/NEJM196201182660301. [DOI] [PubMed] [Google Scholar]
- Holt L. B. The pathology and immunology of Bordetella pertussis infection. J Med Microbiol. 1972 Nov;5(4):407–424. doi: 10.1099/00222615-5-4-407. [DOI] [PubMed] [Google Scholar]
- Kendrick P. L., Eldering G., Dixon M. K., Misner J. Mouse Protection Tests in the Study of Pertussis Vaccine: A Comparative Series Using the Intracerebral Route for Challenge. Am J Public Health Nations Health. 1947 Jul;37(7):803–810. [PMC free article] [PubMed] [Google Scholar]
- Mallory F. B., Hornor A. A., Henderson F. F. The Relation of the Bordet-Gengou Bacillus to the Lesion of Pertussis. J Med Res. 1913 Mar;27(4):391–398.3. [PMC free article] [PubMed] [Google Scholar]
- PILLEMER L., BLUM L., LEPOW I. H. Protective antigen of haemophilus pertussis. Lancet. 1954 Jun 19;266(6825):1257–1260. doi: 10.1016/s0140-6736(54)92770-1. [DOI] [PubMed] [Google Scholar]
- PRESTON N. W., EVANS P. Type-specific immunity against intracerebral pertussis infection in mice. Nature. 1963 Feb 2;197:508–509. doi: 10.1038/197508a0. [DOI] [PubMed] [Google Scholar]
- PRESTON N. W., TE PUNGA W. A. The relation between agglutinin production by pertussis vaccines and their immunising potency in mice. J Pathol Bacteriol. 1959 Jul;78:209–216. doi: 10.1002/path.1700780123. [DOI] [PubMed] [Google Scholar]
- PRESTON N. W. TYPE-SPECIFIC IMMUNITY AGAINST WHOOPING-COUGH. Br Med J. 1963 Sep 21;2(5359):724–726. doi: 10.1136/bmj.2.5359.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston N. W. Potency tests for pertussis vaccines: doubtful value of intracerebral challenge test in mice. J Pathol Bacteriol. 1966 Jan;91(1):173–179. doi: 10.1002/path.1700910121. [DOI] [PubMed] [Google Scholar]
- Preston N. W., Stanbridge T. N. Efficacy of pertussis vaccines: a brighter horizon. Br Med J. 1972 Aug 19;3(5824):448–451. doi: 10.1136/bmj.3.5824.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston N. W. Technical problems in the laboratory diagnosis and prevention of whooping-cough. Lab Pract. 1970 May;19(5):482–486. [PubMed] [Google Scholar]
- Shmilovitz M., Preston N. W., Zaltser H., Cahana A. Whooping cough in northern Israel. Isr J Med Sci. 1972 Dec;8(12):1936–1939. [PubMed] [Google Scholar]


