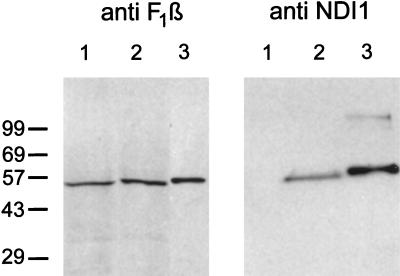
Figure 2.
Immunoblots of mitochondrial fractions from Chinese hamster CCL16-B2 cells by using antibodies against the Ndi1 protein of S. cerevisiae (Right) and the β-subunit of bovine ATP synthase (Left). In both blots, lane 1 is nontransfected cells (100 μg) and lane 2 is NDI1-transfected cells (100 μg). Lane 3 (Left) is bovine mitochondria (1.5 μg). Lane 3 (Right) is the Ndi1 protein (33 ng) containing the leader sequence, which was expressed in and isolated from E. coli. The crude mitochondrial fractions were prepared as follows. The NDI1-transfected and nontransfected cells (approximately 2 × 108 cells) were washed twice with PBS and harvested by trypsinization. The pellet was suspended in 2 ml of isolation buffer containing 210 mM mannitol/70 mM sucrose/1 mM EGTA/5 mM Hepes, pH 7.2/0.2 mM phenylmethanesulfonyl fluoride/0.5% fatty acid-free BSA. The cell suspensions were treated with 0.1 mg/ml of digitonin for 1 min on ice and homogenized in a Dounce homogenizer with a tight pestle (70–100 up/down strokes). The homogenate was centrifuged at 3,000 × g for 5 min at 4°C to remove unbroken cells and nuclei. The supernatant was centrifuged at 10,000 × g for 20 min at 4°C. The pellet was suspended in 0.1 ml of the isolation buffer. This fraction is designated as the crude mitochondrial fraction. Immunoblotting was performed by use of the enhanced chemiluminescence system (Amersham).