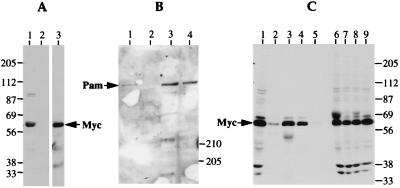
Figure 6.
Myc interacts with Pam in vivo. (A) Binding of Myc to GST-Pam0. GST and GST-Pam0 proteins were incubated with a nuclear extract of CB33-Myc cells, recovered on glutathione agarose beads, and analyzed by Western blotting by using antibody directed against Myc (9E10). Lane 1, binding with GST-Pam0 protein. Lane 2, binding with GST protein. Lane 3, total nuclear extract of CB33-Myc cells. (B) Binding of Pam to GST-Myc1. Purified GST or GST-Myc1 protein was incubated with a nuclear extract of Hela cells, recovered on glutathione agarose beads, and analyzed by Western blotting with an antiserum against Pam. Lane 1, binding with GST-Myc1 protein. Lane 2, binding with GST protein. Lane 3, the supernatant after adsorption of the nuclear extract with GST protein. Lane 4, the supernatant after adsorption of the nuclear extract with GST-Myc1 protein. (C) Coprecipitation of Pam and Myc from nuclear extracts of Hela cells. Extracts were subjected to immunoprecipitation with various antisera. The precipitates were analyzed by Western blotting with antiserum against Myc (9E10). Lane 1, total nuclear extract. Lanes 2–5, immunoprecipitates. Lanes 6–9, supernatants after immunoprecipitation. Lanes 2 and 6, immunoprecipitation with anti-Pam. Lanes 3 and 7, polyclonal anti-Myc from UBI. Lanes 4 and 8, polyclonal anti-Myc prepared by investigators (cv3). Lanes 5 and 9, nonspecific rabbit IgG.