Abstract
Background. Orthotopic liver transplantation (OLT) in adult patients has traditionally been performed using conventional caval reconstruction technique (CV) with veno-venous bypass. Recently, the piggyback technique (PB) without veno-venous bypass has begun to be widely used. The aim of this study was to assess the effect of routine use of PB on OLTs in adult patients. Patients and methods. A retrospective analysis was undertaken of 1067 orthotopic cadaveric whole liver transplantations in adult patients treated between June 1994 and July 2001. PB was used as the routine procedure. Patient demographics, factors including cold ischemia time (CIT), warm ischemia time (WIT), operative time, transfusions, blood loss, and postoperative results were assessed. The effects of clinical factors on graft survival were assessed by univariate and multivariate analyses.In all, 918 transplantations (86%) were performed with PB. Blood transfusion, WIT, and usage of veno-venous bypass were less with PB. Seventy-five (8.3%) cases with PB had refractory ascites following OLT (p=NS). Five venous outflow stenosis cases (0.54%) with PB were noted (p=NS). The liver and renal function during the postoperative periods was similar. Overall 1-, 3-, and 5-year patient survival rates were 85%, 78%, and 72% with PB. Univariate analysis showed that cava reconstruction method, CIT, WIT, amount of transfusion, length of hospital stay, donor age, and tumor presence were significant factors influencing graft survival. Multivariate analysis further reinforced the fact that CIT, donor age, amount of transfusion, and hospital stay were prognostic factors for graft survival. Conclusions. PB can be performed safely in the majority of adult OLTs. Results of OLT with PB are as same as for CV. Liver function, renal function, morbidity, mortality, and patient and graft survival are similar to CV. However, amount of transfusion, WIT, and use of veno-venous bypass are less with PB.
Keywords: piggyback technique, liver transplantation, complication
Introduction
The technique of orthotopic liver transplantation (OLT) has been evolving since its introduction in 1963 1,2,3. Vascular reconstruction has played a major role in this procedure 4,5. During the anhepatic phase there are hemodynamic issues that affect morbidity, mortality, and the entire transplant course. Traditionally, OLT in adult patients has been performed using the conventional caval reconstruction technique (CV) with veno-venous bypass 6. CV involves recipient hepatectomy including the retrohepatic vena cava 1. CV has been a reliable and standard technique. However, hypotension due to the clamping of the major vessels, bleeding from the retroperitoneum, longer vascular reconstruction time, and complication of veno-venous bypass have been the shortcomings of CV 7,8. The piggyback technique (PB) has been used widely in pediatric OLTs as well as OLTs using segmental grafts and living donors. Recently, the advantages of PB have been reported, including lower amount of usage of blood products, shorter operating time, and declining use of veno-venous bypass 8,9,10,11,12,13,14,15,16,17,18,19,20,21. PB involves hepatectomy with preservation of native retrohepatic vena cava 22. This technique also has a few shortcomings, which include outflow obstruction, specifically in the hepatic venous cuff anastomosis 23,24.
The effects of routine use of PB in adult patients on intraoperative results, postoperative results, liver function, renal function, long-term complications, and survival results have yet to be determined in a large series of patients. This study retrospectively evaluated a single center's experience with a large cohort of adult patients who underwent OLT with PB over a 7-year period. PB was used as the first choice of caval reconstruction technique during this period. We examined the effects of routine use of PB on intraoperative and postoperative results as well as long-term outcomes of OLT. We further analyzed the factors that may influence patient and graft survival.
Patients and methods
This is a retrospective review of 1067 consecutive cadaveric adult OLTs performed in 965 patients from June 1994 to July 2001 at the University of Miami/Jackson Memorial Medical Center. PB was used as a routine technique in the majority of cases. In all, 918 transplants (86%) were performed with PB or the modified PB 25,26,27. Modified PB included the suprahepatic cavo-cavoplasty 25,26 and infrahepatic cavo-cavoplasty 27. Of 918 cases, the original piggyback technique was performed in 838 cases (91.3%). Of these 838 cases, 799 (95.3%) were performed using 3 hepatic veins cuff and 39 (4.7%) were performed using 2 hepatic veins cuff due to the size of the donor inferior vena cava. Modified PB were performed for anatomical reasons including the transjugular intrahepatic portal systemic shunt (TIPS) procedure, domino transplantation, or short stump of the donor inferior vena cava 25,26,27. Patient demographics, age, sex, body weight, diagnosis, tumor presence, United Network for Organ Sharing (UNOS) status, retransplantation, donor age, cold ischemia time (CIT) are summarized in Table I. In all, 149 transplants (14%) were performed with CV. The reasons for undertaking CV were as follows: presence of tumors close to the inferior vena cava, presence of the intrahepatic cava, Budd-Chiari syndrome, or technical difficulties including presence of the large caudate lobe or severe inflammation and adhesion between the caudate lobe and the retrohepatic inferior vena cava. The reason for using veno-venous bypass were as follows: hypotension due to intolerance of inferior vena cava clamping, previous TIPS procedure, previous abdominal surgery making dissection in the portal hilum difficult, anatomic reasons including fulminant liver failure without the collateral veins, or intrahepatic inferior vena cava or large caudate lobes.
Table I. Patient demographics and graft characteristics.
| Parameter | Piggyback (n=918) | Conventional (n=149) | p value |
|---|---|---|---|
| Age (years) | 50.8±11.3 | 48.4±13.6 | 0.021873 |
| Sex (male/female) | 573/345 | 104/45 | 0.0827 |
| Body weight (kg) | 79.8±17.6 | 76.4±15.6 | 0.058223 |
| Diagnosis | |||
| Hepatitis C | 412 (44.9%) | 70 (47.0%) | 0.6329 |
| Alcohol | 118 (12.9%) | 12 (8.1%) | 0.0966 |
| Cryptogenic | 85 (9.3%) | 10 (6.7%) | 0.3111 |
| Hepatitis B | 63 (6.9%) | 13 (8.7%) | 0.4124 |
| Fulminant liver failure | 47 (5.1%) | 8 (5.4%) | 0.8984 |
| PSC | 49 (5.3%) | 9 (6.0%) | 0.7257 |
| PBC | 45 (4.9%) | 0 (0%) | 0.0058 |
| AIH | 33 (3.6%) | 0 (0%) | 0.0187 |
| Others | 66 (7.2%) | 27 (18.1%) | 0.0000 |
| Tumor presence | 54 (5.9%) | 48 (32.2%) | 0.0000 |
| Retransplantation | 111 (12.1%) | 25 (16.8%) | 0.1116 |
| UNOS status | |||
| 1 | 126 (13.7%) | 17 (11.4%) | 0.4415 |
| 2 | 486 (52.9%) | 77 (51.7%) | 0.7745 |
| 3 | 306 (33.3%) | 55 (36.9%) | 0.3917 |
| Donor age (years) | 39.3±21.8 | 35.4±17.4 | 0.039468 |
| CIT (min) | 478.4±149.2 | 489.1±146.7 | 0.440740 |
PSC, primary sclerosing cholangitis; PBC, primary biliary cirrhosis; AIH, autoimmune hepatitis; UNOS, United Network for Organ Sharing.
We analyzed the effect of PB on intraoperative data, postoperative data, and laboratory data. Intraoperative data included operative time, warm ischemia time (WIT), intraoperative blood requirement, use of veno-venous bypass, hypotension and surgical complications (Table II). Postoperative data included length of intensive care unit stay, length of hospital stay after the transplant, presence of refractory ascites, cause of death (Table III), graft survival and patient survival (Figures 1 and 2), laboratory data (Table IV), and analysis of prognostic factors for graft survivals (Tables V and VI). Laboratory data on post transplant days 1, 3, 5, and 7 included white blood cell counts, hematocrit, platelet counts, total bilirubin, direct bilirubin, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, serum creatinine, prothrombin time, partial thromboplastin time, and fibrinogen (Table IV).
Table II. Intraoperative results.
| Parameter | Piggyback (n=918) | Conventional (n=149) | p value |
|---|---|---|---|
| Operative time (min) | 607.5±177.8 | 640.6±183.3 | 0.037761 |
| WIT (min) | 34.7±10.7 | 44.9±12.7 | 0.000000 |
| Blood requirement (units) | 13.4±11.5 | 17.6±17.8 | 0.000202 |
| Usage of V-V bypass | |||
| Yes | 181 (19.7%) | 118 (79.2%) | 0.00000 |
| No | 737 (80.3%) | 31 (20.8%) |
WIT, warm ischemic time; V-V, veno-venous.
Table III. Postoperative results.
| Parameter | Piggyback (n=918) | Conventional (n = 149) | p value |
|---|---|---|---|
| ICU stay (days) | 6.8±12.9 | 8.3±12.6 | 0.382271 |
| Hospital stay (days) | 22.1±24.7 | 24.6±29.3 | 0.284841 |
| Refractory ascites | 75 (8.2%) | 8 (5.4%) | 0.2364 |
| Anastomosis stricture | 5 (0.54%) | 1 (0.67%) | 0.8481 |
ICU, intensive care unit.
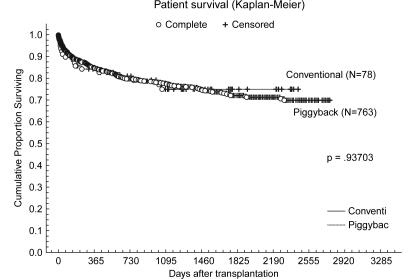
Figure 1. .
Graft survival curves; tumor patients were excluded. Comparison between the piggyback group without tumor (n=865) and the conventional group without tumors (n=101).
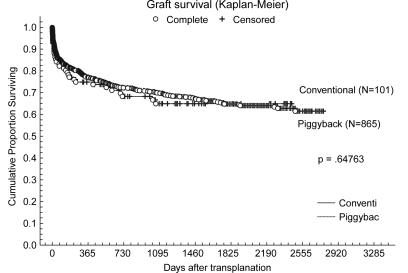
Figure 2. .
Patient survival curves; tumor patients were excluded. Comparison between the piggyback group without tumor (n=763) and the conventional group without tumors (n=78).
Table IV. Laboratory data following OLTs.
| Parameter | Piggyback (n=918) | Conventional (n=149) | p value |
|---|---|---|---|
| Day 1 | |||
| WBC (×103/µl) | 9.71±11.0 | 9.79±8.14 | NS |
| Hct (%) | 33.2±8.82 | 32.5±6.99 | NS |
| Platelet (×103/µl) | 69.4±43.2 | 71.7±41.7 | NS |
| Total bilirubin (mg/dl) | 5.86±5.00 | 5.33±3.63 | NS |
| Direct bilirubin (mg/dl) | 3.92±5.37 | 3.78±7.39 | NS |
| AST (U/L) | 1020.2±1124.7 | 974.2±984.3 | NS |
| ALT (U/L) | 822.9±1012.6 | 812.7±884.8 | NS |
| BUN (mg/dl) | 26.7±18.7 | 25.9±18.7 | NS |
| Cr (mg/dl) | 1.41±2.73 | 1.35±0.85 | NS |
| PT (s) | 18.8±12.2 | 19.1±12.8 | NS |
| PTT (s) | 42.8±17.7 | 43.1±17.2 | NS |
| Fibrinogen (mg/dl) | 201.5±76.9 | 197.0±85.5 | NS |
| Day 3 | |||
| BUN (mg/dl) | 44.8±24.8 | 39.8±23.6 | 0.022311 |
| Cr (mg/dl) | 1.62±1.02 | 1.48±0.91 | NS |
| Day 5 | |||
| BUN (mg/dl) | 46.1±30.7 | 41.7±25.7 | NS |
| Cr (mg/dl) | 1.42±0.96 | 1.33±0.77 | NS |
| Day 7 | |||
| WBC (×103/µl) | 12.2±11.1 | 12.2±9.34 | NS |
| Hct (%) | 32.1±7.42 | 32.4±6.86 | NS |
| Platelet (×103/µl) | 77.8±59.4 | 82.0±59.8 | NS |
| Total bilirubin (mg/dl) | 5.10±8.15 | 4.99±9.49 | NS |
| Direct bilirubin (mg/dl) | 4.10±8.06 | 4.08±9.46 | NS |
| AST (U/L) | 93.9±171.4 | 91.3±150.7 | NS |
| ALT (U/L) | 241.8±251.7 | 236.0±283.9 | NS |
| BUN (mg/dl) | 45.0±32.8 | 42.4±29.2 | NS |
| Cr (mg/dl) | 1.75±6.35 | 1.94±8.00 | NS |
| PT (s) | 16.3±13.6 | 15.6±9.61 | NS |
| PTT (s) | 31.9±16.3 | 31.8±17.5 | NS |
| Fibrinogen (mg/dl) | 287.8±114.5 | 301.4±126.2 | NS |
WBC, white blood cell; Hct, hematocrit; AST, aspartate aminotransferases; ALT, alanine aminotransferases; BUN, blood urea nitrogen; Cr, creatinine; PT, prothrombin time; PTT, partial thromboplastin time.
Table V. Univariate analysis of prognostic factors associated with graft survivals.
| Factors | β | SE | p value | Risk ratio | 95% CI |
|---|---|---|---|---|---|
| Group | −0.292716 | 0.144024 | 0.042121 | 0.746234 | 0.562703–0.989625 |
| CIT (min) | 0.000841 | 0.000349 | 0.015931 | 1.000841 | 1.000157–1.001526 |
| OR time (min) | −0.000126 | 0.000319 | 0.693276 | 0.999874 | 0.999249–1.000499 |
| PRBCs (units) | 0.017973 | 0.003550 | 0.000000 | 1.018135 | 1.011076–1.025244 |
| WIT (min) | 0.010121 | 0.004598 | 0.027743 | 1.010172 | 1.001110–1.019317 |
| Hospital stay (days) | 0.008491 | 0.001068 | 0.000000 | 1.008527 | 1.006418–1.010640 |
| Donor age (years) | 0.006271 | 0.001476 | 0.000021 | 1.006291 | 1.003384–1.009206 |
| Weight (kg) | −0.001199 | 0.001683 | 0.476397 | 0.998802 | 0.995512–1.002102 |
| Tumor presence | 0.352196 | 0.164745 | 0.032539 | 1.422187 | 1.206171–1.676890 |
Group: value for PB group was 1 and value for CV group was 0. Tumor presence: value for patient with tumor was 1 and value for patient without tumor was 0. The other factors were continuous valuable. CIT, cold ischemic time; OR, operation; PRBCs, packed red blood cells; WIT, warm ischemic time.
Table VI. Multivariate analysis of prognostic factors associated with graft survivals.
| Factors | β | SE | p value | Risk ratio | 95% CI |
|---|---|---|---|---|---|
| CIT (min) | 0.000844 | 0.000356 | 0.017700 | 1.000844 | 1.000146–1.001543 |
| PRBCs (units) | 0.012089 | 0.003808 | 0.001503 | 1.012163 | 1.004636–1.019745 |
| Hospital stay (days) | 0.007918 | 0.001155 | 0.000000 | 1.007949 | 1.005670–1.010234 |
| Donor age (years) | 0.005789 | 0.001458 | 0.000072 | 1.005806 | 1.002936–1.008684 |
CIT, cold ischemic time; PRBC, packed red blood cells.
Donors were procured using the standard technique and liver grafts were preserved with University of Wisconsin solution. Tacrolimus and steroids were used as a baseline immunosuppression. Data for cadaveric adult OLTs performed during this period were collected from chart review, our liver transplant database, and the hospital computer system.
Statistical analysis
Data were analyzed using the STATISTICA statistical program (StatSoft, Inc. Tulsa, OK, USA). For numeric data, results are expressed as mean±SD (standard deviation). Numeric data are compared using Student's t test. Nonparametric data are compared using χ2 analysis. Patient and graft survival estimates were obtained using the Kaplan-Meier product limit method. The log rank test was performed for survival analysis. Graft failure was defined as having occurred upon graft removal or patient death. Univariate and multivariate survival analyses were performed using the Cox's proportional hazards regression model. The value for the PB group was 1 and the value for the CV group was 0. The value for patients with tumor (tumor presence) was 1 and the value for patients without tumor was 0. The other factors analyzed for the Cox's proportional hazards regression model were continuous valuable. A probability of p<0.05 was considered statistically significant.
Results
In all, 1067 adult patients underwent OLT during this period. Of the 1067 cases, 918 (86%) operations were performed with PB. The indications and patient demographics are listed in Table I; the indications were similar to other reports. Seventy-eight cases had primary biliary cirrhosis or autoimmune hepatitis during this period. Of the 78 cases, all the cases (100%) were performed with PB. One hundred and two cases had tumors during this period. Of the 102 cases, 54 cases (52.9%) were performed with PB and 48 cases (47.1%) were performed with CV. Of the 918 cases with PB, 737 (80.3%) were performed without veno-veno bypass and 181 (19.7%) were performed with veno-venous bypass. Veno-venous bypass was used according to the surgeon's decision, for anatomic reasons including fulminant liver failure without the collateral veins, or intrahepatic inferior vena cava. Most of the intraoperative and postoperative results with PB were as same as those with CV. However, usage of the veno-venous bypass was less and WIT was shorter (Table II). Average WIT was 34.7 minutes and the average packed red blood cell (PRBC) requirement was 13.4 units. The average length of stay in the intensive care unit was 6.8 days and hospital stay was 22.1 days. Seventy-five patients (8.2%) developed refractory ascites following OLT that required large volume paracentesis. Most of them were associated with nontechnical reasons including recurrent hepatitis C, acute rejection, tumor recurrence, bacterial peritonitis or graft dysfunction. Of the 75 patients, 5 (6%) were associated with the caval anastomosis stricture. Four of them had the refractory ascites resolved by balloon dilatation and have had no further problems. One patient required periodical balloon dilatations. During the follow-up period (1132±819 days), the incidence of refractory ascites was similar to that of with CV (5.4%) in our institution (Table III). Postoperative laboratory data are listed in Table IV. Those data were similar to the CV patients in our institution. Overall 1-, 3-, and 5-year patient survival rates were 85%, 78%, and 72%, respectively (Figure 2). Overall 1-, 3-, and 5-year graft survival rates were 77%, 70%, and 65%, respectively (Figure 1). The results of a univariate analysis for relations between donor age, tumor presence, intraoperative, and postoperative variables and graft survival are shown in Table V.
The cava reconstruction method, CIT, amount of transfusion, WIT, length of hospital stay, donor age, and tumor presence were statistically significant prognostic factors influencing graft survival (p<0.05). The variables that were significant by univariate analysis were subsequently analyzed using multivariate analysis with the Cox proportional hazard model. The results of a multivariate analysis are shown in Table VI. As a result, CIT, donor age, amount of transfusion, and hospital stay were identified as independent prognostic factors for graft survival (p<0.05). Importantly, the cava reconstruction method as an independent marker did not show prognostic impact on graft and patient survival.
Discussion
This retrospective study represents the largest series of patients undergoing OLT using PB from a single center. This study reinforces the following points. Firstly, routine use of PB significantly decreases WIT and blood requirements and use of veno-venous bypass in adult liver transplantation 8,10,11. Secondly, PB was as safe as CV 12. There were similar results for PB and CV as regards postoperative graft function, renal function, patient and graft survival, incidence of outflow obstruction, and late phase refractory ascites following adult OLT.
We believe that surgical bleeding primarily occurs from the numerous collaterals that are encountered in the retroperitoneum. PB does not require the inferior vena cava to be dissected circumferentially, the plane of dissection being between the liver and the cava. Thus, it is obvious that requirement of blood products as well as time required to achieve adequate hemostasis is less with PB.
Thirdly, we can appreciate the fact that the majority of the cases (918, 86%), could be performed with PB. Of further note 737 cases (80.3%) were performed without the veno-venous bypass and 181 cases (19.7%) were performed with the veno-venous bypass depending on the surgeon's decision. Indications for veno-venous bypass were retransplantation, fulminant liver failure, previous abdominal surgery, large caudate lobes, and hypotension during the hepatectomy. Excluding tumor cases and familiar amyloidotic polyneuropathy (FAP) cases for domino transplantation, 918 of 1014 cases (90.5%) were performed with PB in this series.
We incorporated a variety of technical features designed to help us to perform PB safely (Appendix 1). The length of the upper cava of the graft liver was kept short to prevent kinking and outflow obstruction. After finishing the upper cava anastomosis in an average of 10–15 minutes, donor hepatic veins were clamped and vascular clamping of the recipient hepatic vein was opened to prevent compromise of the inferior vena cava flow. Of 918 cases, the original piggyback techniques were performed in 838 cases (91.3%). Of these 838 cases, 799 (95.3%) were performed using 3 hepatic veins cuff and 39 (4.7%) were performed using 2 hepatic veins cuff. Our standard procedure was the piggyback technique using 3 hepatic veins cuff and they had wide patent caval anastomosis. Two hepatic veins cuff was used for anatomic reasons such as anomalous hepatic vein drainage or TIPS procedure. Caval anastomosis should be performed with a wide patency to prevent outflow obstruction. All the pediatric liver transplantations or live donor liver transplantations have been performed using PB. Mass clamping of the porta hepatis was used in patients with altered hilar anatomy due to previous OLT, severe inflammation due to recurrent peritonitis, or abundance of varices around the hilar strictures.
Anastomotic caval stricture was encountered in five cases (0.54%) in the PB group compared with one case in the CV group (0.67%, p=NS). All of them were treated with balloon dilatation of the venous anastomosis. Occlusion of the caval venous return (1.5–2.5%) and hemorrhage (3%) were reported as specific vascular complications related to PB and mortality for those complications was 18% in multicenter studies 23,24. Refractory ascites in the late period was also reported as a complication specific to PB 23. However, this study showed that those complications exist but were rarely related to this technique if three hepatic vein cuffs were used. During the follow-up periods (1132±819 days), there were no significant differences in incidence of refractory ascites (75 cases, 8.2% in the PB group and 8 cases, 5.4% in the CV group).
Modified PB was performed in 80 cases (8.7%) for anatomical reasons including Budd-Chiari syndrome, TIPS procedures or a domino recipient. Modified PB included the suprahepatic cavo-cavoplasty 25,26 and infrahepatic cavo-cavoplasty 27. Temporary porto-caval shunts were performed in 31 cases (3.4%) to prevent the congestion of the intestine due to absence of collateral circulation 20,21. Timing of clamping the portal vein is another concern during PB. The portal vein flows were maintained as much as possible during the hepatectomy in most of the cases. However, in some patients who had enough collateral circulation, early division of the portal vein facilitated the dissection of the liver from the inferior vena cava without hemodynamic compromise. Decisions were made depending on the individual case conditions and the surgeon's experience.
Primary biliary cirrhosis and autoimmune hepatitis were good indications for PB. Of these cases, 78 (100%) were performed with PB. Compared with hepatitis C, hepatitis B, alcohol, and cryptogenic liver cirrhosis, these cases did not have severe adhesion and inflammation between the liver and the retrohepatic cava. Thus, hepatectomy with PB was easier.
In all, 149 cases (14%) were performed with CV. CV is a safe and reliable technique in adult liver transplant patients. Of these 149 cases, 118 (79.2%) were performed with veno-venous bypass and 31 (20.8%) were performed without veno-venous bypass. Forty-eight cases (32.2%) were performed with CV due to the presence of tumors close to the cava. The other 101 (67.8%) cases were performed for anatomic reasons such as intrahepatic cava, huge liver, TIPS procedure, fulminant liver failure, previous abdominal surgery accompanied by dense adhesions and distorted anatomy, and domino liver transplantation. In general, the factors that necessitated the choice of CV were the presence of tumors, the technical difficulties including the intrahepatic cava, large caudate lobe, and severe adhesion between the liver and cava. There were no differences in the background liver disease as a factor to choose CV except for tumor presence. Based on our experience, we use veno-venous bypass in the presence of certain criteria (Appendix 2). However, we believe that the choice of caval reconstruction and use of veno-venous bypass should be left to the judgment and experience of the surgeon.
Choice of surgical technique is known to trigger a chain of events that can affect resource utilization. The advantages of PB, including less use of veno-venous bypass and PRBCs, seem to result in less cost. A significant reduction in hospital charges (mean $23 500) for a patient undergoing PB has been reported 15. In the current healthcare climate, economic benefits of PB may provide additional advantages to choice of caval reconstruction.
This study also shows that postoperative early liver and renal functions of PB were similar to CV following OLT in this series. There were similar laboratory results after OLT with PB in the white blood cell counts, hematocrit, platelet counts, total bilirubin, direct bilirubin, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, serum creatinine, prothrombin time, partial thromboplastin time, and fibrinogen.
Improvements of patient and graft survival in PB are reported in small series, possibly due to shorter WIT, decreased blood loss, and general technical improvements 25. The correlation between blood transfusion and overall survival after liver transplantation has been also reported previously 28. Univariate analysis showed that donor age, CIT, cava reconstruction methods, amount of PRBCs, WIT, hospital stay, and tumor presence have impacts on graft survival. Further multivariate analysis confirmed donor age, CIT, amount of PRBCs and hospital stay as independent prognostic factors. The CV group included more tumor patients (p=0.0000). The presence of tumors in patients might have an influence on the poor survival in CV groups. When we performed the survival analysis of both groups without tumors, the graft and patient survival with PB were as the same as for CV. We demonstrated that PB did not have any negative effect on the graft survivals. CV did not demonstrate a better survival effect for patients with tumors.
Choice of the caval reconstruction depends on the surgeon's experience and the patient's anatomic milieu. The surgeon should be familiar with a variety of options in performing the caval reconstruction. CV has been a safe and reliable technique; however, routine use of PB in this series showed that PB is also a safe and reliable technique and has some advantages of its own.
Although this study has a few limitations, including the fact that it was a retrospective study, we conclude by stating that PB can be performed safely in the majority of adult OLTs. Complications related to outflow obstruction of the graft and refractory ascites existed but were rare if a three hepatic veins cuff was used. Liver function, renal function, morbidity, mortality, and patient and graft survival were not affected by this technique. However, the amount of PRBC transfusion, WIT, and use of veno-venous bypass were less with PB.
Acknowledgments
The authors thank Debbie Weppler, Jeffrey J. Gaynor, Mary Murtha, and Jonathan Andron for their contribution to clinical data analyses.
Appendix 1: Special adjuncts to the piggyback maneuver utilized at the University of Miami
Length of upper cava of the graft liver was kept short to prevent kinking and outflow obstruction.
The clamp on the recipient hepatic veins was released after the upper caval anastomosis was completed and the donor hepatic veins were clamped if necessary. Thus, the blood flow in the recipient IVC is uninterrupted for a long period of time.
Standard procedure of a cuff using three hepatic veins was utilized to maximize the diameter of the caval anastomosis unless there were anatomic variations or TIPS procedures.
Modified piggyback techniques using infrahepatic cavo-cavostomy or suprahepatic cavo-cavostomy were done when the surgeons encountered anatomical issues such as Budd-Chiari syndrome, TIPS procedure or a domino recipient.
Early ligation of portal vein if possible.
Mass clamping of the porta hepatis and arterializing the liver with the infrarenal arterial conduit in patients with altered hilar anatomy due to previous OLT.
Appendix 2: Indications for veno-venous bypass at the University of Miami
Fulminant liver failure.
Large caudate lobes.
Hypotension due to intolerance of IVC clamping.
Previous abdominal surgery making dissection in the portal hilum difficult.
Retransplantation at the late period accompanied by dense adhesions and distorted anatomy.
Previous TIPS procedure.
References
- 1.Starzl TE, Marchioro TL, Von Kaulla KN, Hermann G, Brittain RS, Waddell WR, et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–76. [PMC free article] [PubMed] [Google Scholar]
- 2.Calne RY, Williams R. Liver transplantation in man-I, observations on technique and organization in five cases. BMJ. 1968;4:535–40. doi: 10.1136/bmj.4.5630.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caline RY, Williams R, Dawson JL, Ansell ID, Evans DB, Flute PT, et al. Liver transplantation in man-II, a report of two orthotopic liver transplants in adult recipients. BMJ. 1968;4:541–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein RM, Secrest CL, Klintmalm GB, Husberg BS. Problematic vascular reconstruction in liver transplantation. Part I. Arterial. Surgery. 1990;107:540–3. [PubMed] [Google Scholar]
- 5.Kirsch JP, Howard TK, Klintmalm GB, Husberg BS, Goldstein RM. Problematic vascular reconstruction in liver transplantation. Part II. Portovenous conduits. Surgery. 1990;107:544–8. [PubMed] [Google Scholar]
- 6.Shaw BW, Martin DJ, Marquez JM, Kang YG, Bugbee AC, Jr, Iwatsuki S, et al. Venous bypass in clinical liver transplantation. Ann Surg. 1984;200:524–34. doi: 10.1097/00000658-198410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury GF, Mann ME, Porot MJ, Abdul-Rasool IH, Busuttil RW. Air embolism associated with veno-venous bypass during orthotopic liver transplantation. Anesthesiology. 1987;67:848–51. doi: 10.1097/00000542-198711000-00048. [DOI] [PubMed] [Google Scholar]
- 8.Reddy KS, Johnston TD, Putnam L, Isley M, Ranjan D. Piggyback technique and selective use of veno-venous bypass in adult orthotopic liver transplantation. Clin Transplant. 2000;14:370–4. doi: 10.1034/j.1399-0012.2000.14040202.x. [DOI] [PubMed] [Google Scholar]
- 9.Cherqui D, Lauzet JY, Rotman N, Duvoux C, Dhumeaux D, Julien M, et al. Orthotopic liver transplantation with preservation of the caval and portal flows. Transplantation. 1994;58:793–6. [PubMed] [Google Scholar]
- 10.Jovine E, Mazziotti A, Grazi AL, Ercolani G, Masetti M, Morganti M, et al. Piggy-back versus conventional technique in liver transplantation: report of a randomized trial. Transpl Int. 1997;10:109–12. doi: 10.1007/pl00003824. [DOI] [PubMed] [Google Scholar]
- 11.Busque S, Esquivel CO, Concepcion W, So SK. Experience with the piggyback technique without caval occlusion in adult orthotopic liver transplantation. Transplantation. 1998;65:77–82. doi: 10.1097/00007890-199801150-00015. [DOI] [PubMed] [Google Scholar]
- 12.Fleitas MG, Casanova D, Martino E, Maestre JM, Herres L, Hernanz F, et al. Could the piggyback operation in liver transplantation be routinely used? Arch Surg. 1994;129:842–5. doi: 10.1001/archsurg.1994.01420320068013. [DOI] [PubMed] [Google Scholar]
- 13.Lerut JP, Molle G, Donataccio M, De Kock M, Ciccarelli O, Laterre PF, et al. Cavocaval liver transplantation without venovenous bypass and without temporary portocaval shunting: the ideal technique for adult liver grafting? Transpl Int. 1997;10:171–9. doi: 10.1007/s001470050037. [DOI] [PubMed] [Google Scholar]
- 14.Margarit C, Lázaro JL, Hidalgo E, Balsells J, Murio E, Charco R, et al. Cross-clamping of the three hepatic veins in the piggyback technique is a safe and well tolerated procedure. Transpl Int. 1998;11(Suppl 1):248–50. doi: 10.1007/s001470050471. [DOI] [PubMed] [Google Scholar]
- 15.Hosein Shokouh-Amiri M, Osama Gaber A, Bagous WA, Grewal HP, Hathaway DK, Vera SR, et al. Choice of surgical technique influences perioperative outcomes in liver transplantation. Ann Surg. 2000;231:814–23. doi: 10.1097/00000658-200006000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ducerf C, Rode A, Adham M, De la Roche E, Bizollon T, Baulieux J, et al. Hepatic outflow study after piggyback liver transplantation. Surgery. 1996;120:484–7. doi: 10.1016/s0039-6060(96)80067-5. [DOI] [PubMed] [Google Scholar]
- 17.Hesse UJ, Berrevoet F, Troisi R, Pattyn P, Mortier E, Decruyenaere J, et al. Hepato-venous reconstruction in orthotopic liver transplantation with preservation of the recipients’ inferior vena cava and veno-venous bypass. Langenbecks Arch Surg. 2000;385:350–6. doi: 10.1007/s004230000149. [DOI] [PubMed] [Google Scholar]
- 18.Nery J, Jacque J, Weppler D, Casella J, Luque C, Siquijor A, et al. Routine use of the piggyback technique in pediatric orthotopic liver transplantation. J Pediatr Surg. 1996;31:1644–7. doi: 10.1016/s0022-3468(96)90038-x. [DOI] [PubMed] [Google Scholar]
- 19.Stieber AC. One surgeon's experience with the piggyback versus the standard technique in orthotopic liver transplantation; is one better than the other? Hepatogastroenterology. 1995;42:403–5. [PubMed] [Google Scholar]
- 20.Belghiti J, Noun R, Sauvanet A. Temporary portocaval anastomosis with preservation of caval flow during orthotopic liver transplantation. Am J Surg. 1995;169:277–9. doi: 10.1016/S0002-9610(99)80151-2. [DOI] [PubMed] [Google Scholar]
- 21.Belghiti J, Noun R, Sauvanet A, Durand F, Aschehoug J, Erlinger S, et al. Transplantation for fulminant and subfulminant hepatic failure with preservation of portal and caval flow. Br J Surg. 1995;82:986–9. doi: 10.1002/bjs.1800820741. [DOI] [PubMed] [Google Scholar]
- 22.Tzakis A, Todo S, Starzl TE. Orthotopic liver transplantation with preservation of the inferior vena cava. Ann Surg. 1989;210:649–52. doi: 10.1097/00000658-198911000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parrilla P, Sánchez-Bueno F, Figueras J, Jaurrieta E, Mir J, Margarit C, et al. Analysis of the complications of the piggy-back technique in 1,112 liver transplants. Transplantation. 1999;67:1214–17. doi: 10.1016/s0041-1345(99)00394-2. [DOI] [PubMed] [Google Scholar]
- 24.Navarro F, Moine ML, Fabre JM, Belghiti J, Cherqui D, Adam R, et al. Specific vascular complications of orthotopic liver transplantation with preservation of the retrohepatic vena cava: review of 1361 cases. Transplantation. 1999;68:646–50. doi: 10.1097/00007890-199909150-00009. [DOI] [PubMed] [Google Scholar]
- 25.Gerber DA, Passannante A, Zacks S, Johnson MW, Shrestha R, Fried M, et al. Modified piggyback technique for adult orthotopic liver transplantation. J Am Coll Surg. 2000;191:585–9. doi: 10.1016/s1072-7515(00)00702-x. [DOI] [PubMed] [Google Scholar]
- 26.Wu YM, Voigt M, Rayhill S, Katz D, Chenhsu RY, Schmidt W, et al. Suprahepatic venacavaplasty (cavaplasty) with retrohepatic cava extension in liver transplantation: experience with first 115 cases. Transplantation. 2001;72:1389–94. doi: 10.1097/00007890-200110270-00010. [DOI] [PubMed] [Google Scholar]
- 27.Nishida S, Pinna A, Verzaro R, Levi D, Kato T, Nery JR, et al. Domino liver transplantation with end-to-side infrahepatic vena cavocavostomy. J Am Coll Surg. 2001;192:237–40. doi: 10.1016/s1072-7515(00)00750-x. [DOI] [PubMed] [Google Scholar]
- 28.Palomo Sanchez JC, Jimenez C, Moreno Gonzalez E, Garcia I, Palma F, Loinaz C, et al. Effects of intraoperative blood transfusion on postoperative complications and survival after orthotopic liver transplantation. Hepatogastroenterology. 1998;45:1026–33. [PubMed] [Google Scholar]