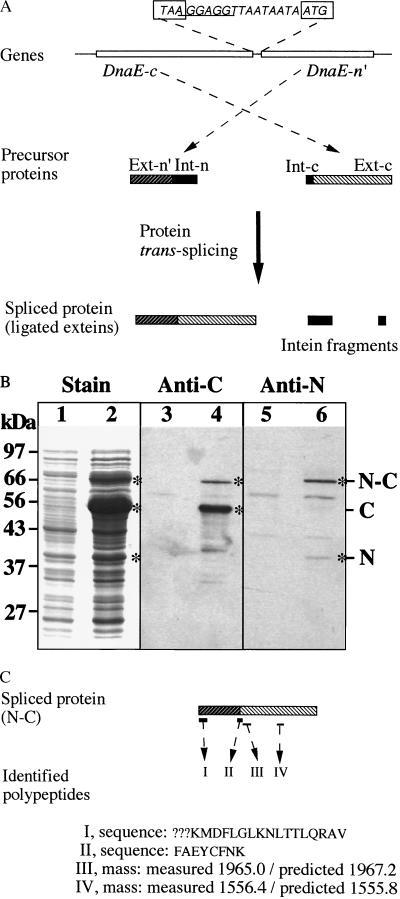
Figure 3.
Protein trans-splicing. The dnaE-n and dnaE-c genes are co-expressed in E. coli cells to observe protein trans-splicing. (A) Schematic illustration. The genes are constructed as a two-gene operon in an expression plasmid vector, with the complete DnaE-c-coding sequence followed by a partial DnaE-n-coding sequence (DnaE-n′). In the intergenic spacer, the termination codon (TAA) of DnaE-c and the initiation codon of DnaE-n′ are boxed, and the Shine-Dalgarno sequence (ribosome-binding site) is underlined. Products of the two genes are shown as precursor proteins, with their extein regions (Ext-n′ and Ext-c) and intein regions (Int-n and Int-c) as indicated. Protein trans-splicing produces a spliced protein and excised intein fragments. (B) Protein gels. Total proteins of uninduced cells (lanes 1, 3, 5) and induced cells (lanes 2, 4, 6) were resolved by SDS/PAGE and visualized by staining (lanes 1 and 2), by Western blotting with anti-C (DnaE-c) antiserum (lanes 3 and 4), or by Western blotting with anti-N (DnaE-n) antiserum (lanes 5 and 6). Positions of precursor proteins (N and C) and the spliced protein (N-C) are marked. (C) Identification of the spliced protein. Peptides I and II were identified by sequencing, and the determined sequences are shown (? marks undetermined residues). Peptides III and IV were identified by mass, with the measured value compared with predicted value.