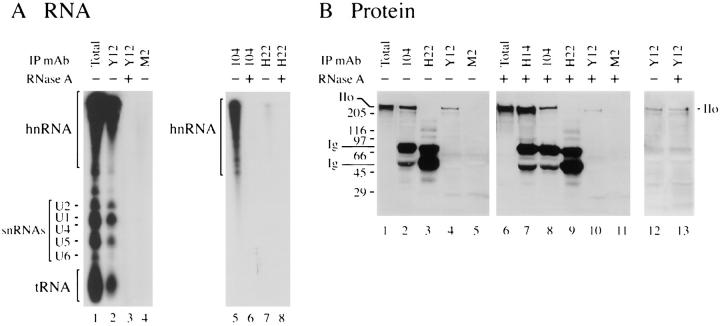
Figure 5.
Pol IIo's association with SR proteins and Sm snRNPs resists RNase digestion. HeLa cells were grown overnight in low phosphate medium supplemented with [32P]orthophosphate (0.5 mCi/ml). The cells were extracted for immunoprecipitation as in Figs. 3 and 4. Immunoprecipitating antibodies include Y12 (anti-Sm snRNP), 104 (anti-SR family proteins), H14 (anti-Pol II), M2 (control IgG), and H22 (control IgM). Immunoprecipitates were washed extensively, and then incubated with RNase (+) or without RNase (−) as described in Materials and Methods. (A) RNA content of immunoprecipitates before and after RNase treatment. Immunoprecipitates prepared from 32P-labeled cells were phenol extracted, ethanol precipitated, rehydrated in loading buffer, and resolved on a 5% (left panel) or 10% (right panel) polyacrylamide/urea gel. The gels were dried and processed for autoradiography. hnRNA, heterogeneous RNAs. U1, U2, U4, U5, and U6, U-rich Sm snRNAs. tRNA, transfer RNA. (B) Proteins in immunoprecipitates before and after RNase treatment. Immunoprecipitates prepared under nonradioactive, but otherwise identical conditions, to those in A. After the RNase digestion step, samples were boiled in SDS sample buffer, subjected to 7% SDS-PAGE electrophoresis, transferred to nitrocellulose, and immunoblotted with anti-Pol II mAb H14. Ig, mouse immunoglobulin chains; IIo, hyperphosphorylated Pol II. Numbers at margins of panels indicate apparent molecular weights in kilodaltons.