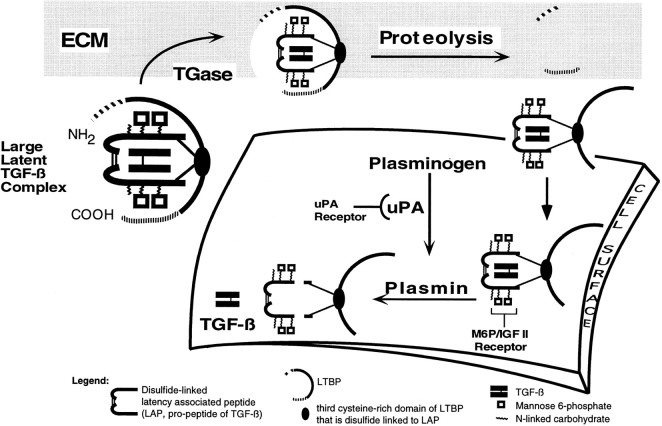
Figure 10.
Model of plasmindependent activation of large latent complex. A diagrammatic representation of large latent complex is presented as covalently interacting with the ECM by transglutaminase (TGase)–dependent cross-linking of LTBP-1 and matrix protein(s). For activation of large latent complex to proceed, large latent complex is solubilized by proteolysis of LTBP-1. It is proposed that the release of large latent complex occurs by cleavage occurring at the amino terminus of LTBP-1 downstream of the TGase reactive site(s). In addition, there may be a second cleavage at the carboxyl terminus of LTBP-1 resulting in matrix release and exposure of mannose 6-phosphate (M6P) residues of LAP involved in localizing latent TGF-β to the cell surface. Proteolytically processed LTBP-1 is proposed to target to the cell surface where cell-associated plasmin cleaves LAP to liberate mature TGF-β from the complex.