Figure 7.
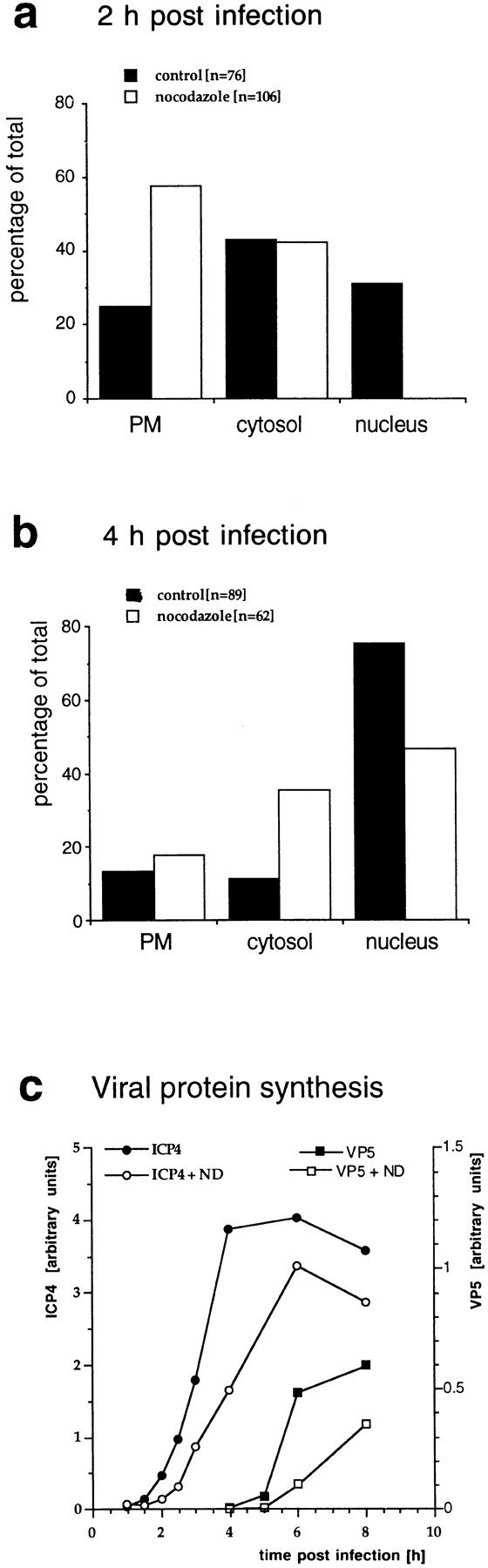
Quantitation of capsid transport and viral protein synthesis in the absence of MTs. Vero cells were infected at an MOI of 170 in the presence of CH and in the absence or presence of nocodazole to depolymerize microtubules. They were fixed at 2 (a) or 4 (b) h postinfection with 1% GA and collected by scraping and pelleting, and Epon sections were prepared. For quantitation, 50 random electron micrographs were obtained in a systematic fashion for each time point and both treatments. The subcellular localization of each cytosolic capsid was determined. (a) At 2 h postinfection in the presence of microtubules, the majority of cytosolic capsids was localized in the cytosol, and a significant fraction had reached the nucleus. In the absence of microtubules, the majority of capsids remained at the plasma membrane (PM), some were in the cytosol, and none had reached the nucleus. (b) At 4 h, >70% of the capsids had reached the nucleus. In the presence of nocodazole, a much smaller fraction was transported to the nucleus, and more capsids remained at the plasma membrane and in the cytosol. (c) Immunoblot. Vero cells were infected at an MOI of 0.5 (without CH!) in the absence or presence of 5 μM nocodazole for various times. The cells were harvested and lysed in 0.5% TX-100 in buffer, the nuclei were pelleted, and the supernatants were analyzed by immunoblotting. To monitor viral protein synthesis, antibodies to ICP4, an early viral protein, and to VP5, a late viral protein, were used. Calnexin, a membrane-bound ER protein, was used as a cellular marker protein. Equal amounts of protein were loaded per lane, and the amount of ICP4 or VP5 was normalized with the amount of calnexin. ICP4 synthesis commences at 2 h PI, whereas VP5 is first detectable at 5 h PI. The amount of ICP4 is maximal after 6 h PI. In contrast, VP5 synthesis increases during the whole time course of the experiment. At all time points, lower amounts of both, ICP4 and VP5, are synthesized in the absence of microtubules.
