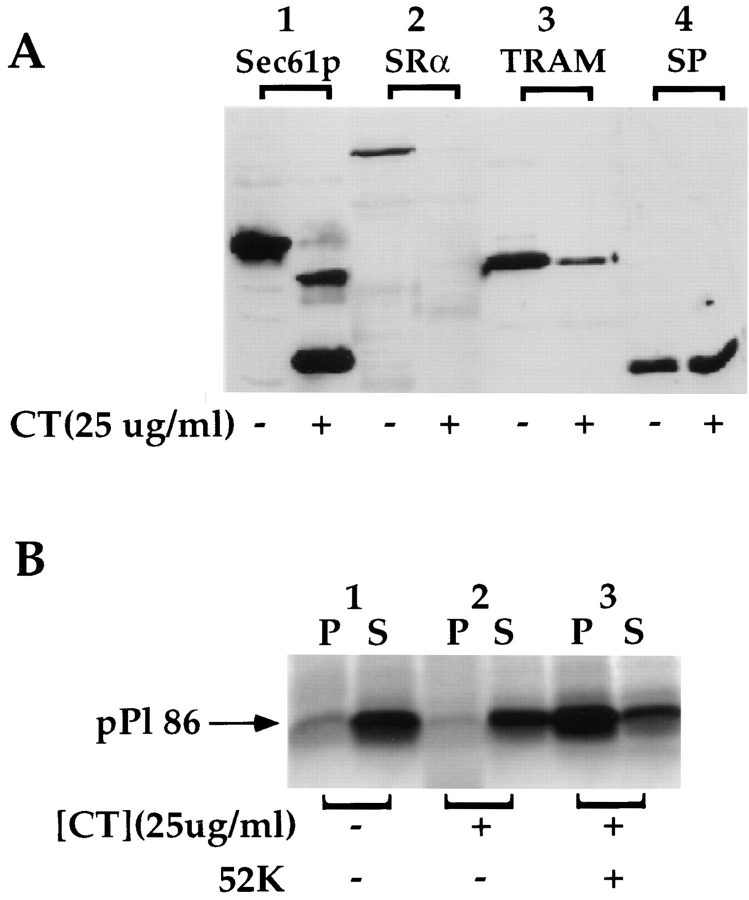
Figure 5.
Proteolysis of Sec61p does not alter ribosome/pPl 86 binding. (A) Untreated and chymotrypsin-treated EKRM were resolved on 12.5% SDS-PAGE gels, and immunoblotted with antibodies directed against Sec61p, SRα, TRAM, and the 22/23-kD subunit of the signal peptidase complex. (B) EKRM were treated with chymotrypsin at 25 μg/ml for 30 min at 4°C, conditions known to yield nearly quantitative digestion of Sec61p. After protease treatment, chymotrypsin-treated EKRM were tested for their ability to support binding of ribosome/pPl 86, either in the presence or absence of the 52-kD fragment of SRα. pPl 86 was translated in the absence of 1.0 eq. EKRM (lane 1), in the presence of 1.0 eq. chymotrypsin-treated EKRM (lane 2), and in the presence of 1.0 eq. chymotrypsin-treated EKRM supplemented with 52 kD SRα fragment (lane 3). The 52-kD SRα fragment was incubated with chymotrypsin-treated EKRM for 30 min at 4°C, before translation.