Figure 4.
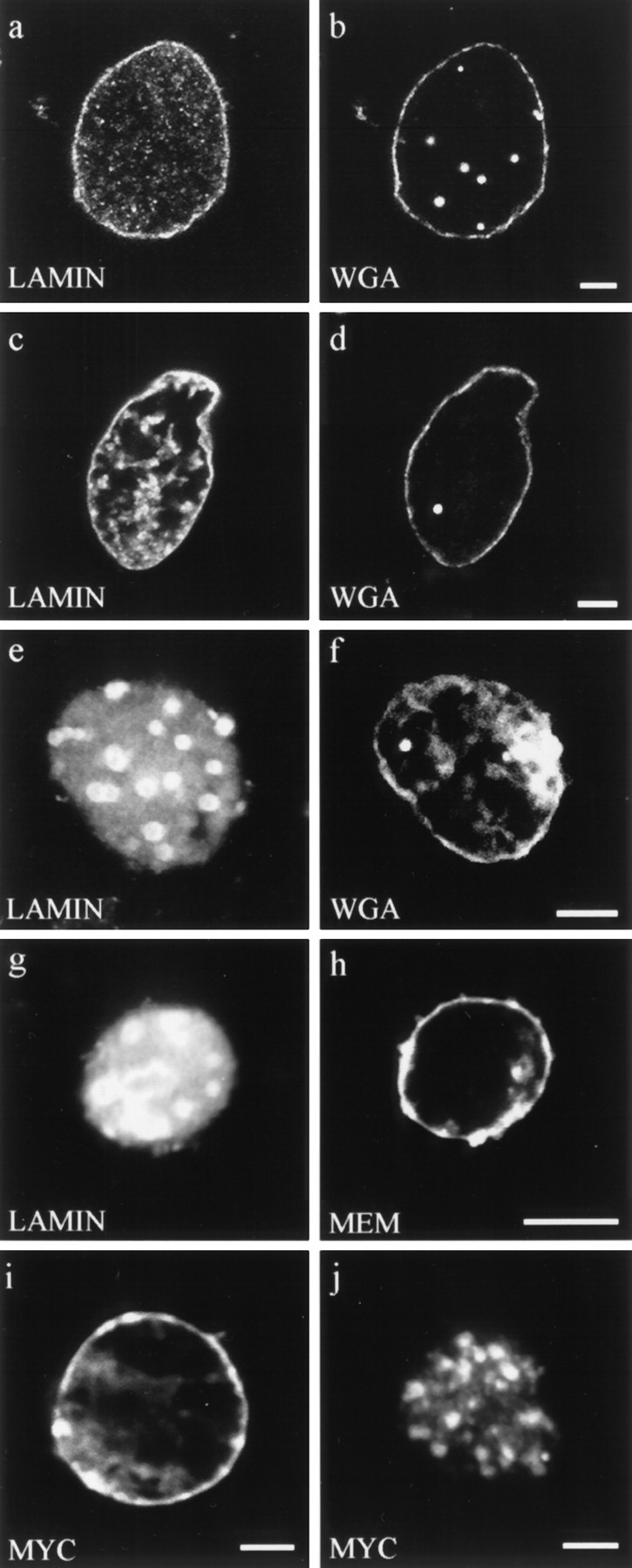
Double label fluorescence observations of nuclei stained for different aspects of nuclear envelope structure and function. (a–f) Nuclei were assembled in interphase extracts containing: (a and b) buffer control, (c and d) lamin A, and (e and f) ΔNLA. Nuclei were stained for (a) lamin B3 or (c and e) human lamin A, and (b, d, and f) the nuclear pore WGA binding proteins using fluorescently tagged WGA. Nuclei assembled under all three conditions appear to have essentially normal distributions of WGA binding proteins at the nuclear periphery. (g and h) Nucleus assembled in the presence of ΔNLA and stained for (g) ΔNLA and (h) the membrane dye DIOC6 (MEM). The nucleus contains a disrupted lamin organization but retains normal membrane staining. Bar, 5 μm. (i and j) Import of wild-type lamin A into (i) buffer control and (j) ΔNLA-disrupted nuclei. The wildtype lamin A was detected using the myc 9E10 epitope antibody (13). Nuclei were assembled with or without ΔNLA, and 90 min after the initiation of assembly, myc-tagged human lamin A was added to the reaction. The nuclei were fixed 20 min later and stained with the myc antibody. Both (i) control and (j) ΔNLAdisrupted nuclei show prominent myc staining, demonstrating that the disrupted nuclei retain the ability to import protein. The majority of the imported protein localizes to the characteristic foci of ΔNLA-disrupted nuclei. Confocal optics showing sections through the mid-region of nuclei. Bar, 5 μm.
