Figure 2.
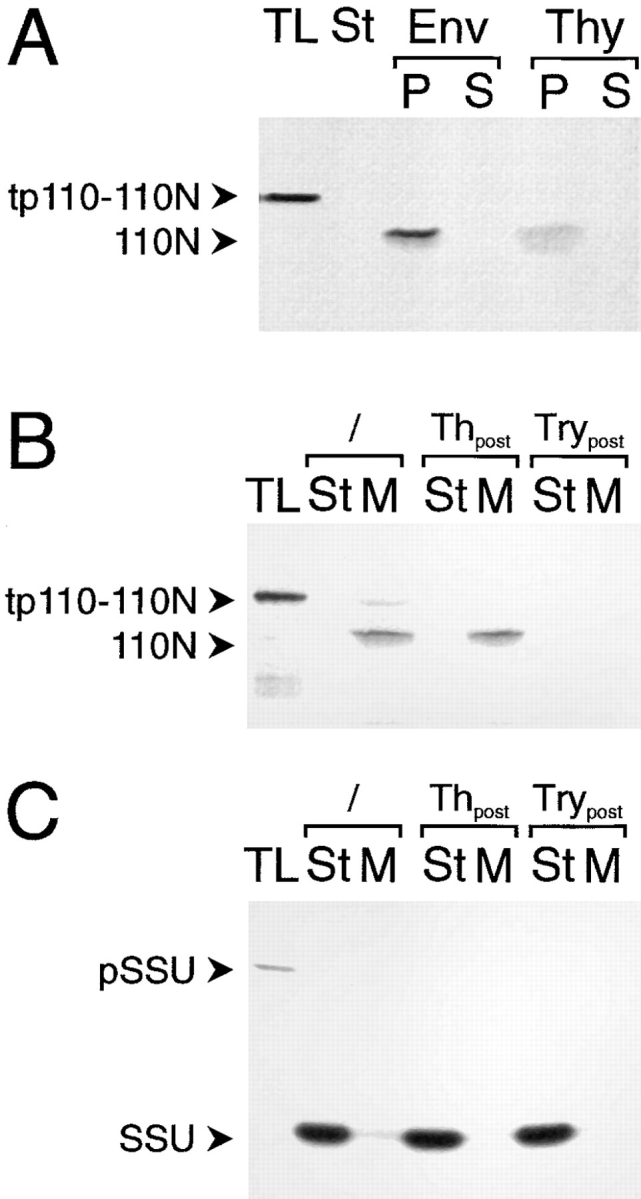
Inner envelope– targeting and insertion information is contained in the NH2 terminus of IEP110. A truncated IEP110 precursor, namely tp110-110N, comprising amino acids 1–269, was used in standard import experiments with purified pea chloroplasts (equivalent to 30 μg chl; A and B). (A) Chloroplasts were separated into different fractions containing either envelope membranes (Env), soluble stroma (St), and thylakoid membranes (Thy). Membranes were subsequently extracted with Na2CO3 at pH 11.5 and further separated into a pellet (P) and soluble fraction (S). (B) Inserted, terminally processed 110N is sensitive to the protease trypsin but not to thermolysin. After completion of a standard import reaction, chloroplasts were either not treated (lanes 2 and 3) or treated with protease (thermolysin [Th], lanes 4 and 5; trypsin [Try], lanes 6 and 7). Organelles were reisolated through a 40% (vol/vol) Percoll cushion washed once. Chlorophyll concentration was determined, and equal amounts of organelles were used for further analysis. Organelles were separated into a soluble stroma (St) and membrane (M) fraction. (C) pSSU is imported into the chloroplast stroma, where the processed mature form is resistant to both proteases, thermolysin and trypsin. All experimental conditions are as decribed in B. 35S-labeled translation product (TL) is shown as an internal standard, 10% of which was added to a standard import reaction. Fluorograms are shown.
