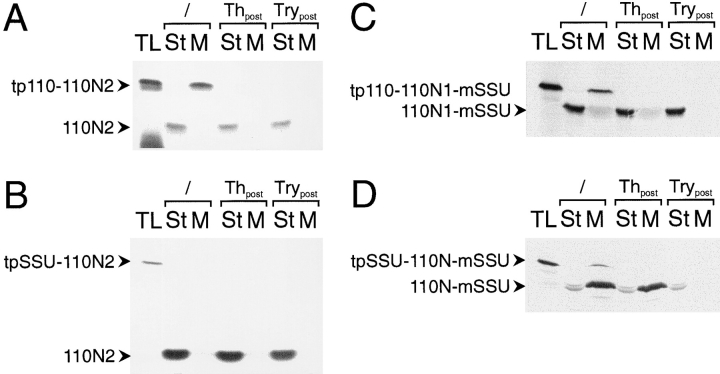
Figure 5.
The N1 and N2 subdomains of IEP110 are both necessary for efficient envelope targeting and insertion. (A and B) amino acids 150–269 of IEP110 were fused to the presequence of either IEP110 or SSU, resulting in tp110-110N2 and tpSSU-110N2, respectively. 35S-labeled translation product was incubated with intact pea chloroplasts (equivalent to 30 μg chl), and the localization of processed, mature 110N2 was tested by chloroplast subfractionation before (lanes 2 and 3) or after treatment with the proteases thermolysin (lanes 4 and 5) or trypsin (lanes 6 and 7). All other experimental conditions are as decribed in Fig. 2. (C) The tp110-110N1-mSSU translation product is imported into pea chloroplasts. Chloroplasts were subfractionated into a soluble stroma phase (lanes 2, 4, and 6) and a membrane fraction (lanes 3, 5, and 7) either before (lanes 2 and 3) or after treatment of the organelles with the proteases thermolysin (lanes 4 and 5) or trypsin (lanes 6 and 7). (D) The tpSSU-110N-mSSU translation product is imported into the chloroplasts under standard conditions. Chloroplasts are fractionated either before or after protease treatment, as outlined above. All experimental conditions and abbreviations are as outlined in Fig. 2.