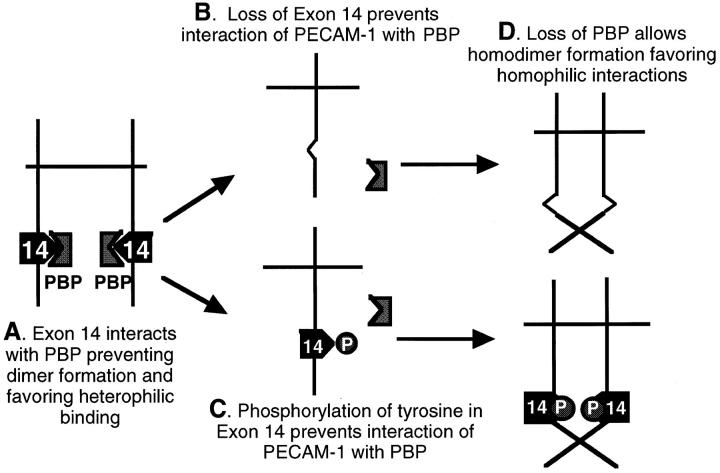
Figure 7.
Proposed mechanism for the role of exon 14 in PECAM-1–dependent adhesion. (A) Under baseline conditions, when full-length PECAM-1 is in its nonphosphorylated state, it interacts with a cytoplasmic protein (a PBP, PECAM binding protein) that binds to a region of the cytoplasmic domain at or near the tyrosine in exon 14. This interaction appears to keep PECAM-1 in a form that favors heterophilic binding. Potential mechanisms for this effect could be induction of a conformation change that is transmitted to the extracellular domain, or possibly maintenance of PECAM-1 in a monomeric, nondimerized form, a conformation that we believe favors heterophilic binding. Either loss of the tyrosine in exon 14 due to deletion/mutation (B) or phosphorylation of this tyrosine (C) inhibits the interaction of PECAM-1 with this PBP that then allows either a conformational change or favors the formation of homodimers that promote homophilic binding (D).