Figure 9.
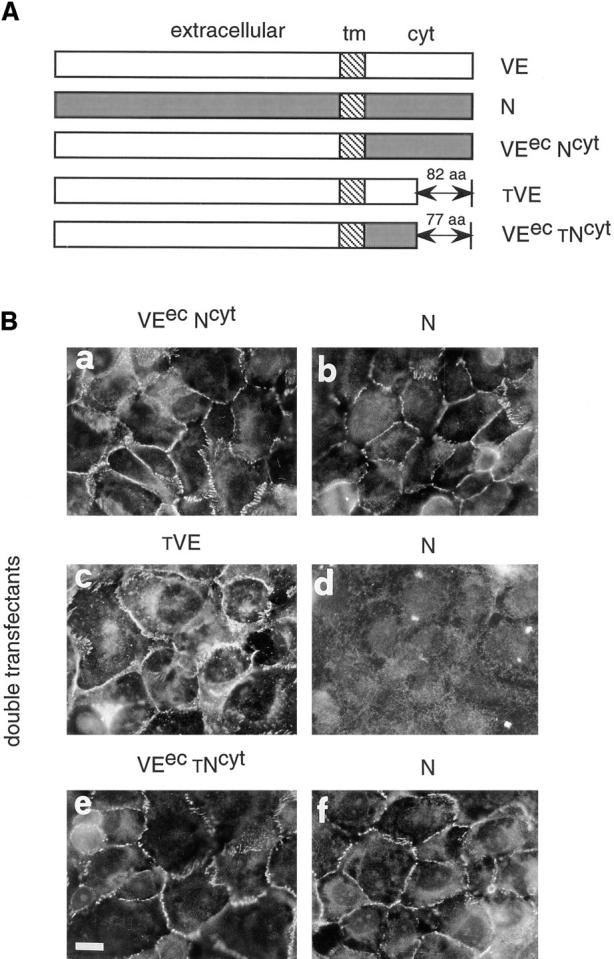
Effect of VE-cadherin mutants and chimeric constructs on N-cadherin clustering at junctions. (A) Schematic representation of the different molecules used for cotransfection: VEecNcyt, a chimeric protein formed by the extracellular region of VE-cadherin and the cytoplasmic domain of N-cadherin; tVE, a truncated form of VE-cadherin lacking the last 82 aas of the COOH terminal end; and VEectNcyt, a chimeric protein formed by the extracellular region of VE-cadherin and a truncated N-cadherin cytoplasmic domain lacking the last 77 aas. (B) Immunofluorescence analysis of typical double-transfected clones. Cells were stained with antibodies directed to the extracellular domain of VE cadherin (BV9 mAb; a, c, and e) or N-cadherin (8C11mAb; b, d, and f). The clones were selected for immunofluorescence studies on the basis of their capacity to express comparable amount of N-cadherin and mutant VE-cadherin constructs by Western blot analysis. In cells cotransfected with VEecNcyt (a), N-cadherin could cluster at intercellular contacts (b). In contrast, when the cells were cotransfected with the tVE mutant (c), N-cadherin was excluded from junctions (d) and this effect was specific because the cells cotransfected with VEec tNcyt (e) mutant shows N-cadherin localization at cell–cell contacts (f). Bar, 30 μm.
