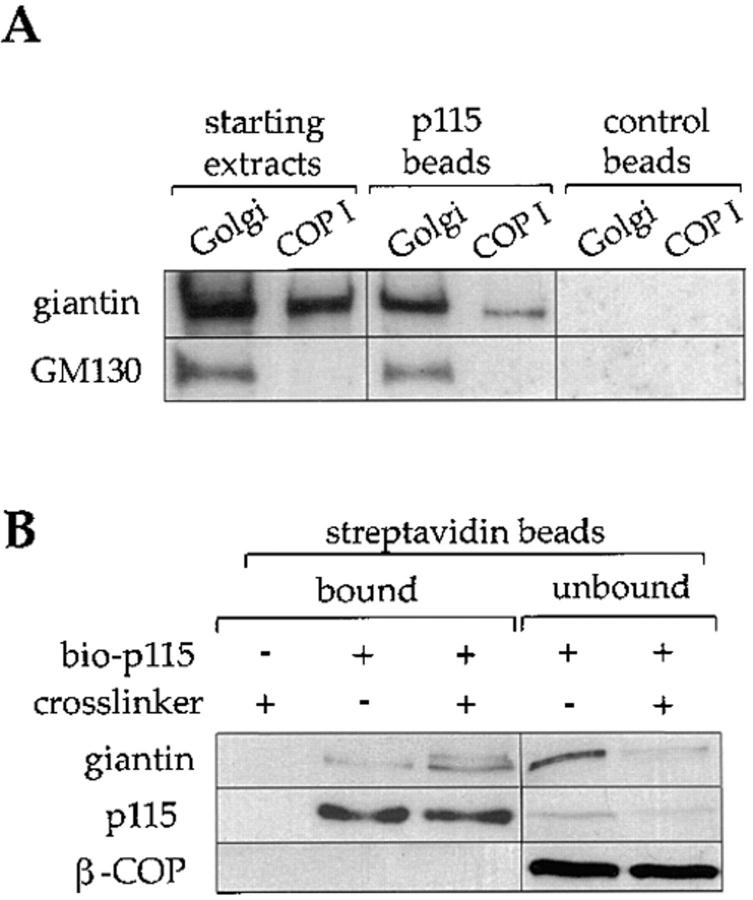
Figure 4.
Biotinylated p115 binds to giantin in COPI vesicles. (A) Triton X-100 extracts of rat liver Golgi membranes or purified COPI vesicles from interphase incubations were incubated with biotinylated p115 immobilized on streptavidin beads, or control beads. Starting extracts (30% of total), and proteins bound to p115 beads or control beads (100% of total) were fractionated by SDS-PAGE, transferred to nitrocellulose, and then probed with antibodies against giantin and GM130. (B) Purified COPI vesicles were incubated with or without biotinylated p115 (bio-p115) for 15 min on ice, the membranes reisolated on a sucrose flotation gradient, and then incubated with or without the cross-linker, DTSSP. After quenching the cross-linker, proteins were extracted with Triton X-100, 0.5 M KCl, and then the extracts were incubated with streptavidin beads. The cross-linker was cleaved by boiling the samples in sample buffer with 50 mM DTT. Bound and unbound fractions were subjected to SDS-PAGE and analyzed by Western blotting, probing with antibodies against giantin, p115, and β-COP. Note that the streptavidin beads quantitatively depleted biotinylated p115 from the extracts.