Figure 9.
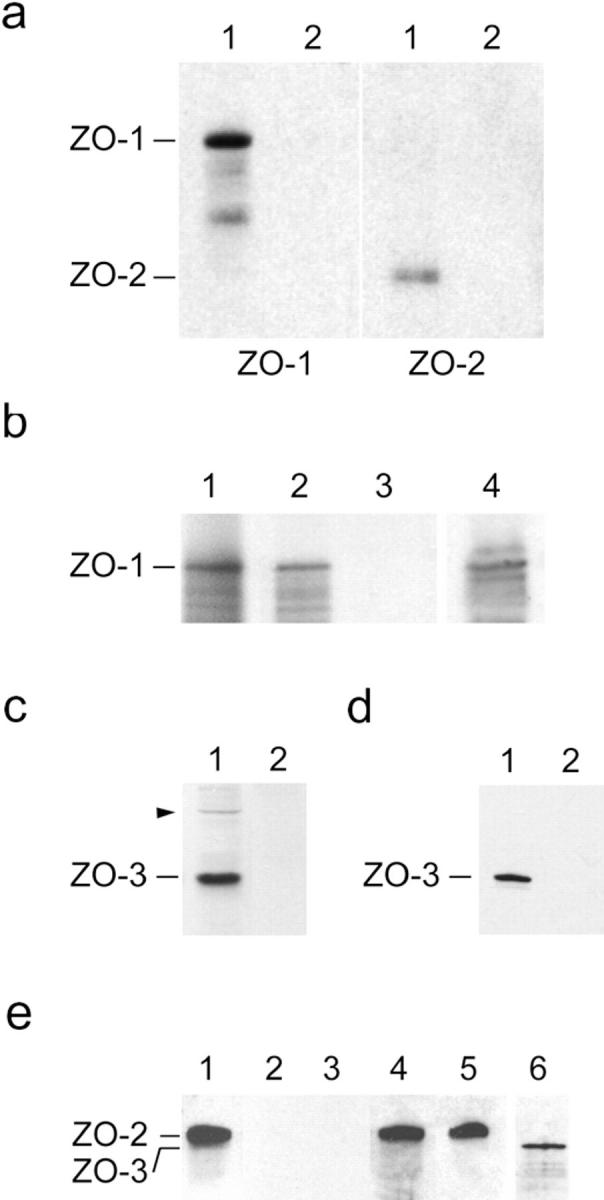
Binding of recombinant ZO-3 to tight junction proteins. (a) ZO-3 binds ZO-1 and ZO-2 from MDCK cell extracts. Affinity resin containing either full-length ZO-3 (lanes 1) or negative control peptide (lanes 2) were incubated with high salt extracts of MDCK cell membranes, washed, solubilized, and immunoblotted for either ZO-1 (left) or ZO-2 (right). The resin containing ZO-3 specifically retains both ZO-1 and ZO-2. (b) ZO-3 binds ZO-1 directly. Radioactively labeled ZO-1 generated by in vitro transcription/translation (lane 1) was incubated with affinity resin containing either full-length ZO-3 (lane 2) or negative control peptide (lane 3). Resin was washed, solubilized, and subjected to SDS-PAGE. The resin containing ZO-3 specifically retains a band which runs at 215 kD. This band was confirmed as ZO-1 by immunoblotting an identical aliquot of the bound material in lane 2 with anti-human ZO-1 antisera (lane 4). Lanes 1–3, autoradiograms; lane 4, ECL. (c) Partial characterization of an anti–ZO-3 antisera. Guinea pig antisera generated against a portion of ZO-3 reacts with a 130-kD band present in whole MDCK cell lysate (lane 1). It also shows faint reactivity with a band that comigrates with ZO-1 (arrowhead). No reaction with MDCK cell proteins was detected with preimmune sera (lane 2). (d) ZO-3 binds directly to the cytoplasmic tail of occludin. Recombinant ZO-3 was incubated with affinity resin containing either the COOH-terminal 148 aa of occludin (lane 1) or negative control peptide (lane 2). Bound material was eluted from washed resin and immunoblotted with anti–ZO-3 antisera. The resin containing occludin specifically retains ZO-3. (e) ZO-3 does not bind to ZO-2. Recombinant ZO-2 (lane 1) was incubated with affinity resin containing ZO-3 (lane 2) or negative control peptide (lane 3). Bound material was eluted from washed resin and immunoblotted with anti–ZO-2 antisera. No binding was detected in either case. Unbound fractions collected from resin containing ZO-3 (lane 4) or negative control peptide (lane 5) were also immunoblotted with anti–ZO-2 antisera. The presence of ZO-3 on the resin of lane 2 was verified by stripping the blot and reprobing with anti–ZO-3 antisera (lane 6).
