Figure 6.
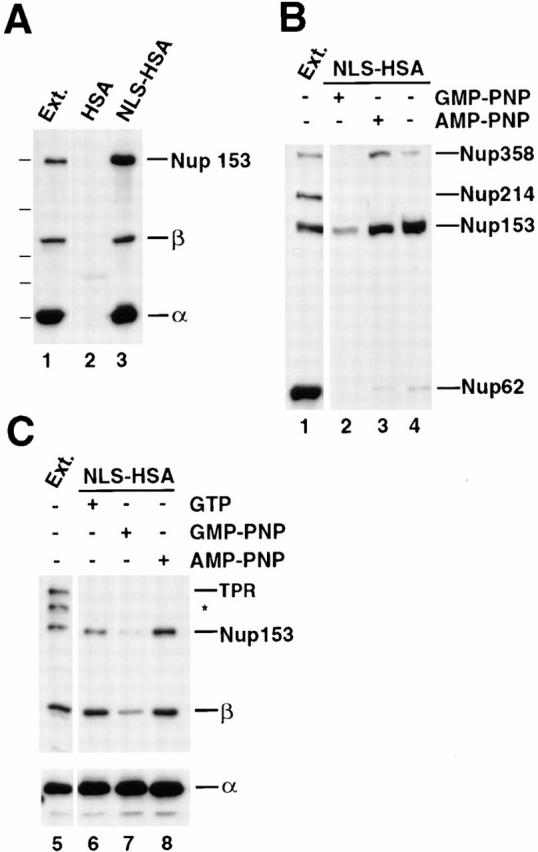
Nup153, but not Tpr, binds to importin α/β heterodimer in the NLS-bound form. (A) Anti-HSA antibody coupled to protein A–Sepharose was added to Xenopus egg extract (diluted 1:100 in buffer ELBS) containing 2.4 μg HSA (lane 2) or 1.9 μg NLS-HSA (lane 3). The proteins that bound to the NLS-HSA/anti-HSA/ Protein A beads were eluted and immunoblotted with a mix of anti–importin α and β and anti-Nup153 (381) antibodies. The proteins present in 0.2 μl of soluble Xenopus egg extract are shown in lane 1 for comparison. Importin α, importin β, and Nup153 all bind to an NLS-HSA affinity column (lane 3), but not to an HSA column (lane 2). (B) GMP-PNP disrupts Nup153 bound to importin α/β– NLS complexes. NLS-HSA/anti-HSA Sepharose beads were added to Xenopus egg extract pretreated with GMP-PNP, AMP-PNP, or no addition (lanes 2–4, respectively). Before bead addition, extracts were diluted with buffer ELBS and supplemented with 1.9 μg of NLS-HSA per aliquot. The NLS-HSA bead mixtures were incubated in extract for 2 h. Then bound proteins were eluted, electrophoresed, and immunoblotted with mAb414 (lanes 1–4). Soluble Xenopus egg extract (0.2 μl) was immunoblotted in parallel (lane 1) to indicate the nucleoporins recognized in the total extract by mAb 414 (Nup358, Nup214, Nup153, and Nup62). Much of the Nup358 and Nup153, but only a trace of Nup62, bind to the NLS-HSA affinity column (lanes 3 and 4). GMP-PNP disrupts the majority of this binding (lane 2), whereas AMP-PNP does not (lane 3). (C) Tpr does not bind to importin α/β–NLS complexes. The experiment in (A) was repeated, and the bound proteins were immunoblotted with a mix of anti-Tpr, anti-Nup153 (381), anti–importin α and β (lanes 5–8). Nup153 and importin α and β bind to the NLS-HSA beads (lane 6), but Tpr does not (lane 6). GMP-PNP releases the importin β and Nup153 from the beads (lane 7), whereas AMP-PNP does not (lane 8). (An asterisk denotes a breakdown product of Tpr.)
