Abstract
The laminin-5 component of the extracellular matrices of certain cultured cells such as the rat epithelial cell line 804G and the human breast epithelial cell MCF-10A is capable of nucleating assembly of cell– matrix adhesive devices called hemidesmosomes when other cells are plated upon them. These matrices also impede cell motility. In contrast, cells plated onto the laminin-5–rich matrices of pp126 epithelial cells fail to assemble hemidesmosomes and are motile. To understand these contradictory phenomena, we have compared the forms of heterotrimeric laminin-5 secreted by 804G and MCF-10A cells with those secreted by pp126 cells, using a panel of laminin-5 subunit-specific antibodies. The α3 subunit of laminin-5 secreted by pp126 cells migrates at 190 kD, whereas that secreted by 804G and MCF-10A cells migrates at 160 kD. The pp126 cell 190-kD α3 chain of laminin-5 can be specifically proteolyzed by plasmin to a 160-kD species at enzyme concentrations that do not apparently effect the laminin-5 β and γ chains. After plasmin treatment, pp126 cell laminin-5 not only impedes cell motility but also becomes competent to nucleate assembly of hemidesmosomes. The possibility that plasmin may play an important role in processing laminin-5 subunits is supported by immunofluorescence analyses that demonstrate colocalization of laminin-5 and plasminogen in the extracellular matrix of MCF-10A and pp126 cells. Whereas tissue-type plasminogen activator (tPA), which converts plasminogen to plasmin, codistributes with laminin-5 in MCF-10A matrix, tPA is not present in pp126 extracellular matrix. Treatment of pp126 laminin-5–rich extracellular matrix with exogenous tPA results in proteolysis of the laminin-5 α3 chain from 190 to 160 kD. In addition, plasminogen and tPA bind laminin-5 in vitro. In summary, we provide evidence that laminin-5 is a multifunctional protein that can act under certain circumstances as a motility and at other times as an adhesive factor. In cells in culture, this functional conversion appears dependent upon and is regulated by tPA and plasminogen.
Epithelial cells are separated from connective tissue by a basement membrane that is composed of a variety of extracellular matrix molecules including proteoglycans, collagen, and laminin isoforms. Together, these proteins create a framework that is essential for maintaining tissue integrity. However, extracellular matrix proteins play more than just a structural role; they also display a diverse set of biological functions that regulate adhesion, migration, proliferation, differentiation, and gene expression of adjacent cells (Roskelly et al., 1995).
Laminin, of which there are at least 10 isoforms, is a major component of basement membranes, and has been shown to mediate cell–matrix attachment, gene expression, tyrosine phosphorylation of cellular proteins, and branching morphogenesis (Tryggvason, 1993; Timpl and Brown, 1994; Streuli et al., 1995; Malinda and Kleinman, 1996; Stahl et al., 1997). The expression patterns of the laminin isoforms are tissue specific. The laminin-5 isoform (nicein, epiligrin, and kalinin) is abundant in transitional epithelium, stratified squamous epithelia, lung mucosa, and other epithelial glands (Kallunki et al., 1992; Stahl et al., 1997). Laminin-5 is a heterotrimer consisting of α3, β3, and γ2 subunits that associate via large α helical regions to produce a cruciform-shaped molecule (Rousselle et al., 1991; Baker et al., 1996a ). Laminin-5 is synthesized initially as a 460-kD molecule that undergoes specific processing to a smaller form after being secreted into the extracellular matrix (Marinkovich et al., 1992; Vailly et al., 1994; Matsui et al., 1995a ). The size reduction is a result of processing the α3 and γ2 subunits from 190–200 to 160 kD and from 155 to 105 kD, respectively (Marinkovich et al., 1992; Vailly et al., 1994; Matsui et al., 1995a ). The identity of the proteases involved in such proteolytic events has not been described previously.
In a number of studies, it has been demonstrated that laminin-5 produced by 804G cells can nucleate the assembly of hemidesmosomes in squamous cell carcinoma (SCC)112, human adult calcium-transformed (HaCaT), pp126, and normal human keratinocyte (NHEK) cells as well as in corneal epithelial cells maintained in vitro (Langhofer et al., 1993; Hormia et al., 1995; Baker et al., 1996a ,b; Tamura et al., 1997; El-Ghannam et al., 1998). However, SCC12, HaCaT, pp126, and NHEK cells themselves secrete laminin-5, which is incapable of supporting the assembly of hemidesmosomes, suggesting that structural differences between laminin-5 molecules may regulate their functions. The current data demonstrate that the electrophoretic mobility of the α3 chain of laminin-5 in the matrix of these cell types is distinct. Furthermore, proteolytic processing of the α3 subunit of laminin-5 in the extracellular matrix is crucial for hemidesmosome formation. We also present evidence that processing of the α3 subunit of laminin-5 can be mediated by a plasmin-dependent mechanism involving tissue-type plasminogen activator (tPA)–catalyzed plasminogen activation. A model is proposed that could explain the role of laminin-5 processing in hemidesmosome assembly.
Materials and Methods
Protein Preparations and Other Chemicals
Plasminogen and plasmin were purified as previously described (Stack et al., 1993, 1994; Stack and Pizzo, 1994). Mast cell tryptase was provided by D. Johnson (East Tennessee State University, Johnson City, TN; Little and Johnson, 1995). Purified two-chain tPA was the gift of H. Berger (Wellcome Research Laboratories, Research Triangle Park, NC). Urinary-type plasminogen activator (uPA) was purchased from Calbiochem-Novabiochem Corp. (La Jolla, CA). The serine proteinase inhibitor, dichloroisocoumarin, was purchased from Sigma Chemical Co. (St. Louis, MO). Matrix metalloproteinase-2 (MMP-2, gelatinase A) and MMP-9 (gelatinase B) were obtained from the serum-free conditioned medium of epithelial ovarian carcinoma cells as previously described or were the gift of H. Nagase (University of Kansas, Lawrence, KA) (Young et al., 1996). Laminin-1 was a gift from N. Chilukuri (Northwestern University Medical School, Chicago, IL).
For affinity purification of pp126 laminin-5, tissue culture plastic was coated with 50 μg/ml GB3 antibody, a mouse monoclonal antibody against the γ2 chain of human laminin-5, in 10 mM Tris, pH 7.4, overnight at 4°C (Verrando et al., 1987; Matsui et al., 1995b ). Dishes were rinsed briefly and then incubated with conditioned medium from pp126 cells for 1 h at 37°C. The dishes were then washed in 20 mM Tris, pH 7.4, containing 250 mM NaCl, and then followed by three washes in 10 mM Tris, pH 7.4. To assess the purity of the laminin-5 prepared by this procedure, confluent dishes of pp126 cells were radiolabeled overnight with 50 μCi/ml of [35S]PRO-MIX cell label (Amersham Corp., Arlington Heights, IL). The labeled, conditioned medium of the pp126 cells was then overlaid on the GB3 antibody-coated dishes as above. After washing, the material “captured” by the antibody was solubilized in SDS-PAGE sample buffer, proteins were resolved by SDS-PAGE, and then the resulting gel was prepared for autoradiography (see below). For overlay studies, human laminin-5 was provided by Desmos Inc. (San Diego, CA).
Cell Culture and Preparation of Laminin-5 Matrices
Michigan Cancer Foundation MCF-10A cells and 804G cells were maintained as described previously (Riddelle et al., 1991; Stahl et al., 1997). NHEK (purchased from Clonetics Corp., San Diego, CA), SCC12, and pp126 cells, a gift from D. Oda (University of Washington, Seattle, WA) were maintained in the serum-free growth medium Keratinocyte-SFM (GIBCO BRL, Gaithersburg, MD) supplemented with 20 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 5 ng/ml EGF, and 50 μg/ ml bovine pituitary extract (Oda et al., 1996). MCF-10A, pp126, SCC12, NHEK, and 804G cell matrix were prepared as described previously (Gospodarowicz, 1984; Langhofer et al., 1993). In experiments where various proteases were used, pp126 cells were removed from their matrix with ammonium hydroxide as previously described. Then the matrix was extensively washed in PBS and ∼50 μg of matrix was incubated with 1 ml of PBS containing plasmin at concentrations of 0.01, 0.1, and 1 μg/ml for 90 min at 37°C or 50 μg of matrix was treated with 1 ml of PBS containing 5 μg/ml of either MMP-2 or MMP-9 overnight at 37°C. In some studies, 50 μg of pp126 cell matrix was treated overnight at 37°C with 1 ml of PBS containing either 5 or 10 μg/ml tPA. In all cases, dichloroisocoumarin was then added to the treated matrix at 10 μg/ml for 15 min at room temperature. After washing with PBS, the treated matrices were solubilized in sample buffer consisting of 8 M urea, 1% sodium dodecyl sulfate in 10 mM Tris-HCl, pH 6.8, and 15% β-mercaptoethanol.
SDS-PAGE, Western Immunoblotting, and Overlay Assays
Protein preparations were separated by the method of Laemmli (1970) in 6% acrylamide gels. In cases where radiolabeled proteins were resolved by SDS-PAGE, gels were dried and then exposed to X-Omat Imaging Film (Eastman Kodak Co., Rochester, NY). For immunoblotting, separated proteins were transferred to nitrocellulose according to standard procedures (Harlow and Lane, 1988). The nitrocellulose membranes were processed for blotting as described in Zackroff et al. (1984). Blots were developed either using chloronaphthol as a colorimetric reagent, or using the LumiGlo chemiluminescence kit (Kirkegaard and Perry Laboratories, Inc., Gaithersburg, MD). For overlay assays, 1 ng of protein was dotted onto nitrocellulose, which was then blocked in PBS containing 0.2% fish gelatin. The membrane was subsequently incubated overnight with 20 μg/ml of either tPA, uPA, or plasminogen, at 4°C with vigorous shaking. The nitrocellulose was blocked in 5% milk in PBS and then processed for immunoblotting.
Antibodies
GB3 antibody against the γ2 chain of human laminin-5 was purchased from Harlan Sprague Dawley Inc. (Indianapolis, IN) (Verrando et al., 1987; Matsui et al., 1995b ). For production of a rabbit serum against the human γ2 chain, a clone encoding amino acids 522–722 of human laminin-5 γ2 chain was identified in a λgt11 keratinocyte expression library (Clontech Laboratories Inc., Palo Alto, CA) as detailed in Langhofer et al. (1993). A 25-ml culture of Y1090 was grown at 37°C in shaking suspension to an OD of 0.7 and was subsequently inoculated with a 108 phage containing the laminin-5 γ2 insert. After 1 h, isopropyl B-p-thiogalactopyranoside was added to a concentration of 1 mM and then incubated for an additional 3 h. Cells were pelleted and then resuspended in gel sample buffer (8 M urea, 1% SDS in 10 mM Tris-HCl, pH 6.8, 5% β-mercaptoethanol). The resulting protein sample was then simultaneously processed for SDS-PAGE with a protein sample derived from non-isopropyl B-p-thiogalactopyranoside–induced cells. After staining of the gel, a prominent protein migrating at ∼140 kD (i.e., a portion of laminin-5 γ2 chain fused with β-galactosidase) was observed exclusively in the induced cells. This polypeptide was excised, rinsed in PBS, and then used to immunize a rabbit for polyclonal antibody production (Harlow and Lane, 1988). Blood was collected from the rabbit at 3-wk intervals.
For production of serum antibodies against the COOH-terminal region of the human laminin-5 α3 subunit, a 535-base pair HindIII/XhoI cDNA fragment encoding amino acid residues 1561–1713 of the G5 domain of the α3 subunit was subcloned into the HindIII and XhoI sites of the pET32b vector (Novagen, Inc., Madison, WI) and then transfected into DE3α cells (Ryan et al., 1994). A histidine (His) fusion protein was induced and then the cells expressing the fusion protein were extracted in SDS buffer, as described above. The fusion protein was identified using a His-HRP probe (SuperSignal HisProbe Western blotting kit; Pierce Chemical Co., Rockford, IL) and on an SDS-PAGE gel after protein staining in Coomassie brilliant blue (Sigma Chemical Co., St. Louis, MO). The protein was excised from the gel and then the gel pieces were homogenized and subsequently injected into BALB/C mice for generation of mouse antisera. One such serum (Cta3) was used in the course of these studies, although all of the sera showed the same immunoblotting reactivities.
Clone 17, a mouse monoclonal antibody specific for the β3 chain of human laminin-5, was purchased from Transduction Laboratories (Lexington, KY). The mouse monoclonal antibody 10B5, which recognizes the α3 subunit of rat and human laminin-5, was generated using 804G laminin-5 as immunogen as described in Langhofer et al. (1993). The mouse monoclonal inhibitory antibody P3H9-2 against human laminin-5 and monoclonal antibody MAB 1921 against the β1 laminin subunit were purchased from Chemicon International, Inc. (Temecula, CA). PAM-3, a mouse monoclonal antibody against human tPA, and monoclonal antibody 394 against human uPA, were purchased from American Diagnostica Inc. (Greenwich, CT). Antihuman plasminogen rabbit antiserum was described in Stack et al. (1993, 1994) and Stack and Pizzo (1994).
Immunofluorescence
MCF-10A and pp126 cells were maintained on glass coverslips for 24–48 h, permeabilized in acetone at −20°C for 2 min, and then air dried. Coverslips were incubated with a mix of primary antibodies diluted in PBS at 37°C in a humid chamber for 1 h, washed three times in PBS, and then incubated for an additional hour at 37°C with the appropriate mix of secondary antibodies conjugated to rhodamine and FITC. Mounted glass coverslips were viewed using a laser scanning confocal microscope (model LSM10; Zeiss Inc., Thornwood, NY). Images were stored on optical discs (Sony Corp., Park Ridge, NJ) and printed on a Tektronix printer (model Phaser IISDX; Tektronix, Wilsonville, OR).
Electron Microscopy
Cells maintained on tissue culture plastic or on matrix for 36 h were fixed in 1% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2, for a minimum of 30 min. Fixed preparations were washed three times in 0.1 M sodium cacodylate buffer, pH 7.2, and then postfixed in 1% osmium tetroxide containing 0.8% potassium ferricyanide. Samples were then stained with uranyl acetate, dehydrated six times in ethanol, and then embedded in Epon-Araldite resin (Tousimis Corp., Rockville, MD). Sections were cut perpendicular to the substratum and viewed on an electron microscope (model 1220; JEOL USA, Inc., Peabody, MA) at 60 kV (Riddelle et al., 1991). Morphometric analyses were performed using the software of this microscope.
Motility Assay
Cells were plated in media containing 20 mM Hepes onto various substrata for 1 h. They were subsequently maintained at 37°C and then viewed by phase-contrast microscopy using a Nikon Diaphot system (Tokyo, Japan). The field was photographed every 5 min over a 2-h period with a chilled charge-coupled device camera (Hamamatsu Photonic Systems Corp., Park Ridge, IL). The location of each cell was translated to numerical coordinates using the public domain National Institutes of Health Image Program (Bethesda, MD), and motility for each cell was calculated as displacement in micrometers from the starting point to the ending point. An average of 30 cells was monitored for each trial.
Results
The α3 Subunit of Laminin-5 Is Processed Differently in Diverse Cell Types
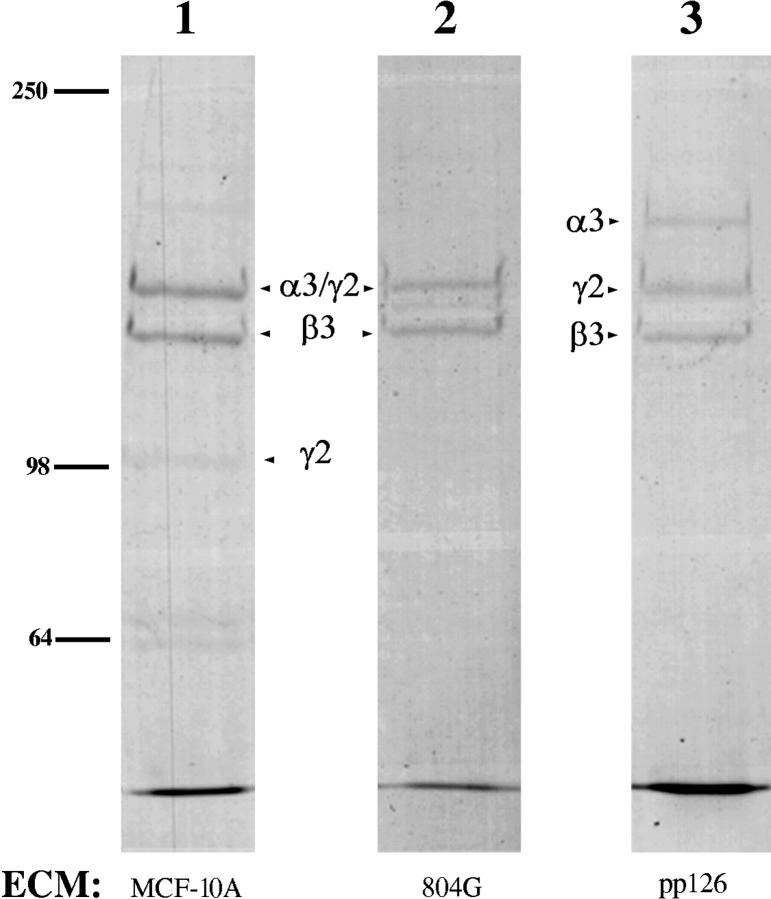
The rat epithelial cell line 804G and the human breast epithelial cell line MCF-10A are unusual in that both assemble cell–matrix attachment devices called hemidesmosomes when maintained in tissue culture (Riddelle et al., 1991; Stahl et al., 1997). Recent data indicate that this property is dependent upon the laminin-5 secreted by these cells (Baker et al., 1996a ; Stahl et al., 1997; Tamura et al., 1997). However, laminin-5 is expressed by many other cell types, such as the squamous cell carcinoma line SCC12 and the transformed oral epithelial cell line called pp126, which do not assemble hemidesmosomes under normal conditions (Langhofer et al., 1993; Baker et al., 1996a ; Tamura et al., 1997). In addition to expressing laminin-5, both SCC12 and pp126 cells express all the major known protein components of hemidesmosomes, yet only assemble hemidesmosomes when plated onto laminin-5 secreted by 804G cells or MCF-10A cells (Langhofer et al., 1993; Baker et al., 1996a ; Tamura et al., 1997; Jones, J.C.R., unpublished observations). The latter finding suggests the possibility that distinct structural and functional forms of laminin-5 may be expressed by different cell types. Thus, we have analyzed the subunit composition of laminin-5 secreted by 804G, MCF-10A, pp126, and SCC12 cells, as well as a “normal” cell population (NHEK), by Western immunoblotting using a panel of antibodies against specific laminin-5 subunits. For our studies, we prepared the matrix secreted by these cell types using a procedure developed by Gospodarowicz (1984). Laminin-5 subunits are the major constituents of these matrices (Fig. 1 shows the protein profiles of 804G, MCF-10A, and pp126 matrices).
Figure 1.
The extracellular matrices of MCF-10A, 804G, and pp126 cells (lanes 1–3) were prepared according to Gospodarowicz (1984). Approximately 15 μg of each sample was run on the lane of a 6% SDS-PAGE gel that was subsequently stained with Coomassie brilliant blue. The α3, β3, and γ2 chains of laminin-5 are indicated (see Fig. 2 for the corresponding immunoblots). Note that in MCF-10A and 804G cell matrices the 160-kD form of the α3 chain and 155-kD form of the γ2 chain migrate at about the same molecular weight. In addition, the 105-kD form of the γ2 chain is stained poorly by Coomassie brilliant blue. Molecular weight standards are indicated to the left.
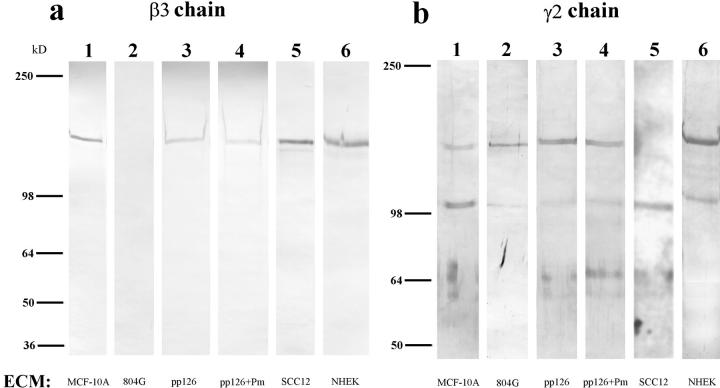
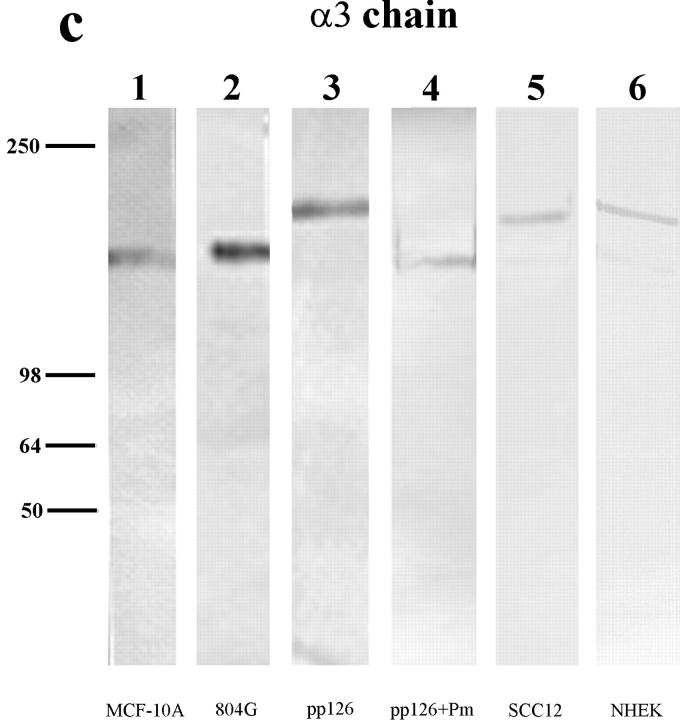
An antibody against the β3 subunit of laminin-5 recognizes a band migrating at 145 kD present in MCF-10A as well as SCC12, NHEK, and pp126 cell matrices (Fig. 2 a). This particular monoclonal antibody does not recognize rat material, hence the lack of reactivity with the 804G cell matrix preparation. A polyclonal antibody recognizing the γ2 subunit of human laminin-5, which displays cross-reactivity with the rat homologue, detects 155- and 105-kD polypeptides in the matrices of MCF-10A, 804G, pp126, and NHEK cells but only a 105-kD species in the matrix of SCC12 cells (Fig. 2 b). For the analysis of the α chain of laminin-5, we made use of a monoclonal antibody, 10B5, which was prepared against laminin-5 in the extracellular matrix of 804G cells (Langhofer et al., 1993; Baker et al., 1996a ). 10B5 specifically recognizes the α3 chain of rat laminin-5 and displays cross-reactivity with the α3 subunit of the human homologue (Fig. 2 c). The α3 subunits of pp126, SCC12, and NHEK cell laminin-5 migrate at 190 kD, identical to the reported size of the unprocessed α3 chain of laminin-5 (Fig. 2 c) (Marinkovich et al., 1992; Matsui et al., 1995a ). The appearance of the unprocessed α3 chain of laminin-5 in NHEK matrix has not previously been noted (Marinkovich et al., 1992; Matsui et al., 1995a ). In contrast, in the matrices of MCF-10A and 804G cells the α3 chain migrates at 160 kD, similar to the molecular weight of the processed α3 chain subunit (Fig. 2 c) (Marinkovich et al., 1992; Matsui et al., 1995a ). Similar results are obtained using two other monoclonal antibodies against the human α3 subunit (results not shown).
Figure 2.
The laminin-5 subunit compositions of the extracellular matrices (ECM) of MCF-10A, 804G, pp126, SCC12, and NHEK cells are shown (lanes 1–6). Approximately 10 μg of matrix protein was run on each lane of a 6% gel, transferred to nitrocellulose, and then processed for immunoblotting using laminin-5 subunit– specific antibody preparations. (a) The laminin-5 β3 chain was identified using the monoclonal antibody clone 17. Clone 17 antibody recognizes a protein of 145 kD in all of the human matrices but shows no reactivity with 804G cell matrix (compare lane 2 with lanes 1, 3, 5, and 6). (b) Antiserum J20, against the laminin-5 γ2 chain, recognizes 155-kD polypeptides in matrix preparations derived from MCF-10A, 804G, pp126, and NHEK cells (lanes 1, 2, 3, and 6), as well as a 105-kD species in the matrices of MCF-10A, 804G, SCC12, and NHEK cells (lanes 1, 2, 5, and 6). (c) The laminin-5 α3 chain is identified using the mouse monoclonal antibody 10B5, which recognizes a polypeptide migrating at 160 kD in 804G and MCF-10A cell matrix (lanes 1 and 2), and a protein of 190 kD in the matrices of pp126, NHEK, and SCC12 cells (lanes 3, 5, and 6).Approximately 50 μg of the matrix of pp126 cells was treated for 90 min with a 1-ml PBS solution containing plasmin (+Pm) at a concentration of 1 μg/ml and then the treated matrix preparation was probed with the β3, γ2, and α3 subunit-specific antibodies (lane 4 in each blot). The mobility of the β3 and γ2 subunits are unaffected by such treatment (compare b, lanes 3 and 4), whereas the α3 subunit migrates at 160 kD, compared with 190 kD in the untreated matrix (compare c, lanes 3 and 4). Molecular weight standards are indicated to the left.
These results indicate that there is no obvious correlation between the sizes of the γ2 or β3 subunits of laminin-5 and the ability of a cell to assemble a hemidesmosome. In contrast, the matrix of those cells (MCF-10A and 804G) that assemble hemidesmosomes contains processed α3 subunits, whereas matrix of those cells (pp126, SCC12, and NHEK) that do not assemble hemidesmosomes contains unprocessed α3 subunits. Thus, we next tested several proteinases that are known to cleave extracellular matrix proteins for their ability to alter the electrophoretic mobility of the laminin-5 α3 subunit of pp126, SCC12, and NHEK cells. For these studies, we chose proteinases that are associated with these cells (i.e., plasmin, MMP2, and MMP9) (Stack, M.S., unpublished observations). We describe only those results using pp126 cells since the results are identical to those using the other cell lines. MMP-2 and MMP-9 at enzyme/substrate ratios of ∼1:10 exhibit no obvious effect on the α3 subunit of pp126 matrix (data not shown). We also treated ∼50 μg of pp126 matrix with 1 ml of PBS containing the proteinase plasmin at concentrations of 0.01, 0.1, and 1 μg/ml for 90 min. Plasmin at concentrations of 0.01 and 0.1 μg/ml has no obvious effect on the subunits of laminin-5 (result not shown). However, after treatment of pp126 matrix with plasmin at 1 μg/ml, the α3 subunit is converted to a 160-kD species, as shown by Western immunoblotting with 10B5 antibody (Fig. 2 c). This treatment does not induce proteolysis of the β3 and γ2 subunits (Fig. 2, a and b). In addition, a 90-min treatment of pp126 matrix with 1 μg/ml of mast cell tryptase, which, like plasmin, is a trypsin-like serine proteinase, also converts the 190-kD α3 subunit to a 160-kD species (result not shown).
Where Does Plasmin Cleave the 190-kD Form of the α3 Subunit?
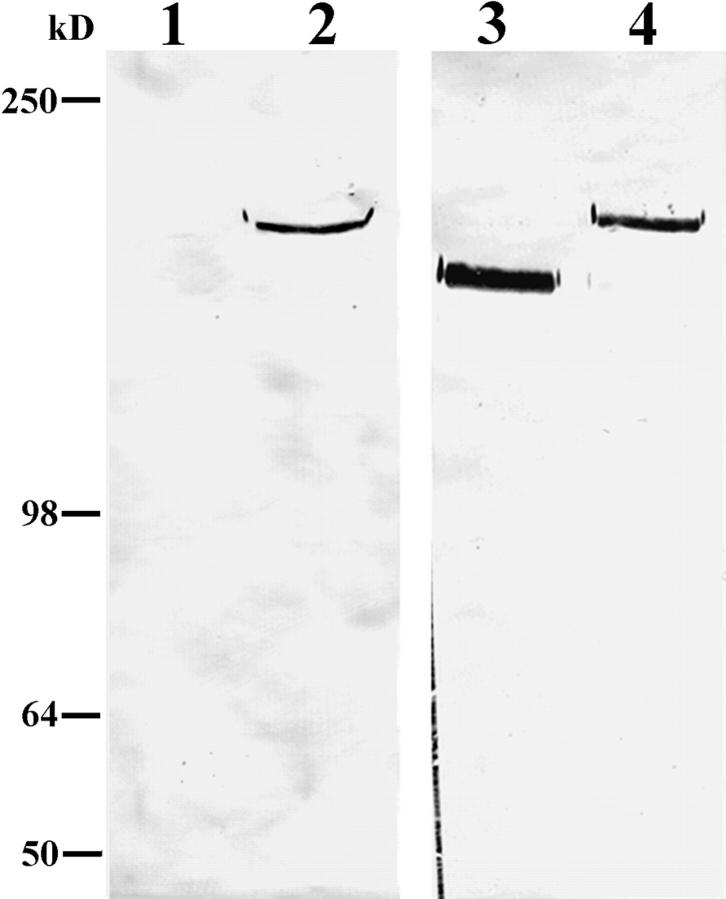
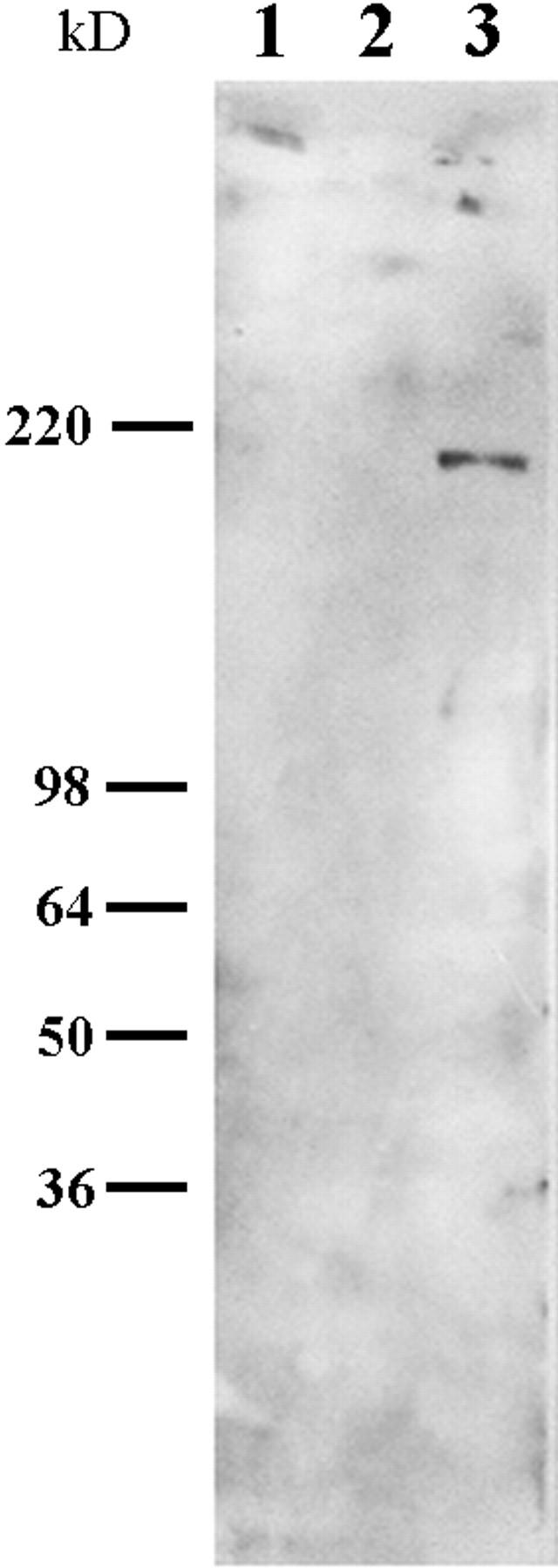
To test the possibility that plasmin cleavage occurs towards the COOH terminus of the α3 subunit of laminin-5, we prepared an antiserum (Cta3) against residues 1561– 1713 at the COOH terminus of the α3 subunit. The Cta3 serum contains antibodies that show reactivity with the 190-kD unprocessed form of the α3 subunit present in pp126 matrix, but which fail to show reactivity with any species in MCF-10A matrix (Fig. 3). In contrast, the 10B5 monoclonal antibody recognizes the unprocessed 190-kD α3 subunit in pp126 matrix as well as the processed 160-kD species in MCF-10A matrix in a comparable immunoblot (Fig. 3). Although this study does not rule out the possibility there may be a plasmin cleavage site close to the NH2 terminus of the molecule, it provides direct evidence that plasmin can cleave the 190-kD α3 subunit towards its COOH terminus.
Figure 3.
Approximately 10 μg of either MCF-10A (lanes 1 and 3) or pp126 cell (lanes 2 and 4) ECM was run on lanes of a 6% gel, transferred to nitrocellulose, and then processed for immunoblotting using either a mouse serum (Cta3) raised against residues 1561–1713 at the COOH terminus of the α3 subunit of laminin-5 (lanes 1 and 2) or the α3 subunit monoclonal antibody 10B5 (lanes 3 and 4). Antiserum Cta3 recognizes a 190-kD protein in pp126 ECM (lane 2), but does not show reactivity with any polypeptide in MCF-10A ECM (lane 1). The 190-kD species in pp126 ECM is also recognized by 10B5 antibodies (lane 4). However, unlike the Cta3 serum, 10B5 antibodies recognize a 160-kD protein in MCF-10A ECM (lane 3). Molecular weight markers are indicated at the left of the immunoblots.
Functional Consequences of Processing of the α3 Subunit of Laminin-5
Cell Motility.
The specific, limited proteolytic cleavage of the α3 chain of pp126 cell laminin-5 from 190 to 160 kD suggests that plasmin-mediated proteolysis may have functional consequences for this matrix ligand. Thus, we assessed the motility of SCC12 cells plated on the laminin- 5–rich matrix of pp126 cells relative to MCF-10A matrix. SCC12 cells plated onto MCF-10A matrix exhibit significantly lower motility after 2 h compared with SCC12 cells plated onto pp126 matrix (Fig. 4). However, SCC12 cells plated onto plasmin-modified pp126 matrix display a 2.5– 3-fold decrease in motility, similar to that observed on MCF-10A matrix (Fig. 4).
Figure 4.
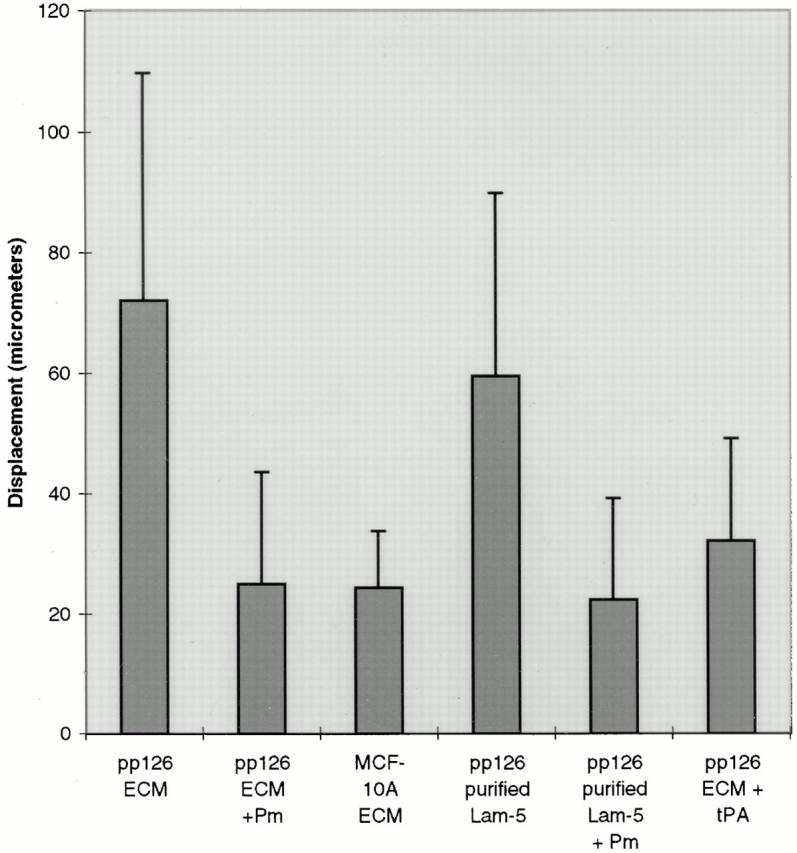
SCC12 cells were plated onto pp126 ECM, pp126 ECM that had been treated with plasmin (Pm) (1 μg/ml for 90 min), MCF-10A ECM, affinity-purified pp126 laminin-5, affinity-purified pp126 laminin-5 treated with plasmin (Pm) (1 μg/ml for 90 min), or pp126 ECM treated with 10 μg/ml tPA overnight. After 1 h the motility of the SCC12 cells was assayed by video microscopy and then quantitated as the average of the total displacements of each cell over a 2-h period. For each substrate, we performed at least three trials in which a total of at least ninety cells were evaluated. Note that SCC12 cells show similar motility on pp126 ECM and affinity-purified pp126 laminin-5. They show less motility on plasmin-treated pp126 ECM, plasmin-modified purified pp126 laminin-5, MCF-10A ECM, and pp126 ECM treated with tPA. Error bars indicate standard deviations.
Since there is evidence that laminin-5 is complexed with other laminin isoforms such as laminin-6, we were concerned that other laminin variants may play a role in the motility phenomena we detail (Champliaud et al., 1996). However, pp126 cell matrix contains little or no detectable laminin-6 as assessed by immunoblotting with a monoclonal antibody probe against the β1 laminin subunit (Fig. 5). In addition, we have assessed the motility of SCC12 cells on affinity-purified pp126 cell laminin-5. To prepare the purified laminin-5, GB3 antibody, immobilized to a cell culture dish, was used to capture laminin-5 from the conditioned medium of radiolabeled pp126 cells (Fig. 6). For some studies, the affinity-purified material was treated for 90 min with plasmin at 1 μg/ml. By SDS-PAGE, the affinity-purified untreated pp126 cell laminin-5 consists of three distinct polypeptides of 190, 145, and 105 kD, representing the α3, β2, and γ2 chains, respectively (Fig. 6). In the plasmin-modified material, the α3 chain migrates at ∼160 kD, whereas the β2 and γ2 chains show no obvious change in their electrophoretic mobility (Fig. 6). These results were confirmed by immunoblotting (data not shown).
Figure 5.
Approximately 10 μg of MCF-10A and pp126 ECM and 5 μg of laminin-1 were processed for SDS-PAGE and then separated polypeptides were transferred to nitrocellulose. The nitrocellulose sheet was then subjected to immunoblotting using a monoclonal antibody against the β1 subunit of laminin. This antibody recognizes a 200-kD protein in laminin-1 but no obvious polypeptides in MCF-10A or pp126 ECM.
Figure 6.
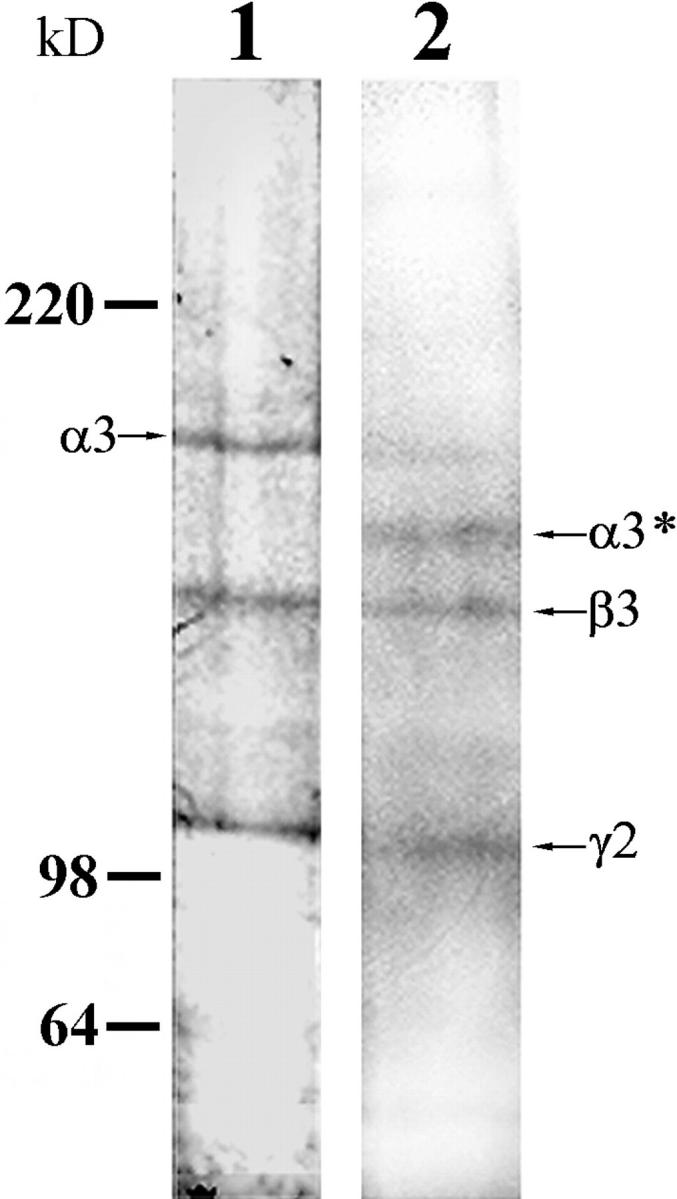
Radiolabeled laminin-5 was captured by the laminin-5 γ2 chain antibody GB3 from the conditioned medium of pp126 cells. One sample was left untreated, whereas the other was digested with 1 μg/ml plasmin for 90 min. Approximately 2 μg of both preparations were then processed for SDS-PAGE and then the gel dried and was exposed to film. Note that the untreated material consists of three prominent polypeptides of 190-, 145- and 105-kD molecular weight (the α, β and γ chains of laminin-5, respectively), whereas in the plasmin-modified material there are major polypeptides of 160 (processed α3 subunit, indicated as α3*), 145, and 100 kD. Note that there is a minor amount of the 190-kD unprocessed α subunit in the plasmin-modified preparation. Molecular weights are indicated to the left of the gel profiles.
SCC12 cells were plated onto both untreated, affinity-purified pp126 cell laminin-5 as well as the plasmin-modified purified laminin-5, and their motility was compared. The 2.4-fold higher motility of SCC12 cells on affinity-purified laminin-5 compared with that on plasmin-modified purified laminin-5 shows significance at P < 0.008 as determined using the nonparametric analysis of variance Mann–Whitney U test (Fig. 4).
Hemidesmosome Assembly.
Laminin-5 is the extracellular ligand of the integrin pairs α6β4 and α3β1 (Carter et al., 1991; Niessen et al., 1994). The precise physiological role of α3β1 integrin–laminin-5 ligation is unknown, whereas the α6β4 integrin–laminin-5 complex forms the core of hemidesmosomes (Stepp et al., 1990; Jones et al., 1994; Borradori and Sonnenberg, 1996; Green and Jones, 1996). Therefore, we investigated whether SCC12 cells are induced to assemble hemidesmosomes on four distinct pp126 cell-derived laminin-5 substrates: untreated pp126 cell laminin-5–rich matrix, plasmin-modified pp126 laminin-5–rich matrix, affinity-purified pp126 laminin-5, and plasmin-modified affinity-purified pp126 laminin-5. SCC12 cells were plated onto these matrices and then after 24 h the samples were processed for electron microscopy. For each matrix, we evaluated hemidesmosome assembly in at least 15 cells in at least two different trials. The results for one complete trial are presented in Table I and representative images are provided in Fig. 7. SCC12 cells maintained on unmodified pp126 matrix and affinity-purified pp126 laminin-5 assemble few hemidesmosomes at their basal surface and those that occur appear immature (Table I). We define an immature hemidesmosome as an electron-dense structure, located along the cell–substrate interface, that has a poorly developed cytoplasmic plaque lacking a trilayered appearance and obvious keratin bundle association (Langhofer et al., 1993; Fig. 7). SCC12 cells plated onto plasmin-modified laminin-5 preparations readily assemble mature hemidesmosomes (Fig. 7, a and b; Table I). These hemidesmosomes possess triangular-shaped, trilayered, electron-dense cytoplasmic plaques and are associated with the keratin intermediate filament cytoskeleton (Fig. 7, a and b). These data support the conclusion that plasmin processing of pp126 cell-derived laminin-5 activates the ability of laminin-5 to nucleate assembly of hemidesmosomes. To provide further confirmation that laminin-5 is involved in nucleation of mature hemidesmosome assembly in this system, we also assessed hemidesmosome assembly in SCC12 cells maintained for 24 h on plasmin-modified pp126 matrix as well as plasmin-modified affinity- purified pp126 laminin-5, both of which had been treated with the laminin-5–blocking antibody 1947. Antibody treatment considerably reduces the formation of mature hemidesmosomes in SCC12 cells maintained on such antibody-treated substrates (Fig. 7 c; Table I).
Table I.
Hemidesmosome Assembly in SCC12 Cells Maintained for 24 H on Various Matrices
| Matrix | n of cells evaluated | Immature HDs | Immature HDs/100 microns | Mature HDs | Mature HDs/100 microns | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| pp126 cell ECM | 19 | 34 | 12.72 | 3 | 1.12 | |||||
| pp126 cell ECM + plasmin | 18 | 23 | 7.66 | 128 | 42.64 | |||||
| pp126 cell ECM + plasmin + 1947 mAb | 19 | 33 | 13.5 | 24 | 9.82 | |||||
| Affinity-purified laminin-5 | 19 | 37 | 9.94 | 0 | 0 | |||||
| Affinity-purified laminin-5 + plasmin | 20 | 23 | 6.24 | 53 | 14.38 | |||||
| Affinity-purified laminin-5 + plasmin + 1947 mAb | 15 | 26 | 8.37 | 7 | 2.25 |
At 24 h after seeding onto the indicated matrices, SCC12 cells were processed for electron microscopy. Thin sections of the cells were prepared perpendicular to their substrate- attached surfaces. The numbers of mature and immature hemidesmosomes were evaluated over a known distance. In two series of studies, the matrix upon which the cells were plated was preincubated with the laminin-5 function-inhibiting antibody 1947. The greater number of hemidesmosomes assembled by SCC12 cells on plasmin-treated pp126 ECM versus plasmin-treated affinity-purified laminin-5 is most likely because there is more laminin-5 in cell-deposited matrix than can be captured by GB3 antibody onto a substrate. HD, hemidesmosome; ECM, extracellular matrix.
Figure 7.
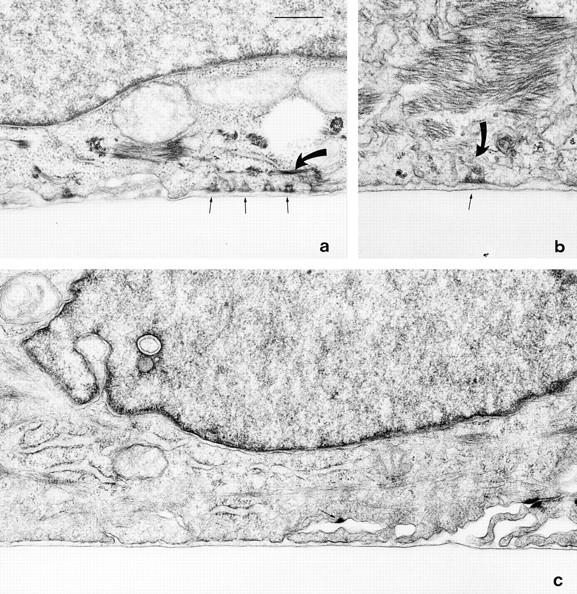
SCC12 cells were maintained for 24 h on plasmin-modified (1 μg/ml for 90 min) pp126 matrix (a), plasmin-modified purified pp126 laminin-5 (b), and plasmin-modified pp126- derived laminin-5 that had been incubated for 30 min at 37°C with 50 μg/ml of the laminin-5 function-inhibiting antibody 1947 before addition of the cells (c). The SCC12 cells were then processed for electron microscopy and cross-sections of the cells were prepared. In a and b, several hemidesmosomes in the SCC12 cells are observed at sites of cell– matrix association (arrows). Each has a triangular, trilayered plaque that is associated with intermediate filaments (curved arrows in a and b). c, the SCC12 cell has assembled no obvious hemidesmosomes along regions of cell–matrix interaction. The arrow indicates one hemidesmosome-like structure that is not associated with the substrate. This structure may even be a half-desmosome. a and c are at the same magnification. Together, these results provide evidence that plasmin-modified pp126 cell laminin-5 is capable of inducing the assembly of hemidesmosomes. Bars: (a and c) 500 nm; (b) 200 nm.
Plasminogen and tPA Interaction with Laminin-5
In Vivo Association.
The apparent ability of plasmin to modify the structure of laminin-5 and the resulting functional consequences on cell behavior raise the question of how these events may be regulated in our culture systems. Plasmin is generated by cleavage of the proenzyme plasminogen, which is present in serum and found in association with extracellular matrices. The cleavage of plasminogen to produce the functional enzyme plasmin is mediated by either of two so-called plasminogen activators designated tPA and uPA (Wun, 1988). Since colocalization of enzyme and substrate is an important regulatory property governing matrix remodeling, we determined whether plasminogen and either tPA or uPA are associated in vivo with laminin-5 (Ranby, 1982; Stack et al., 1995).
MCF-10A and pp126 cells were processed for double- label immunofluorescence microscopy using an antiserum raised against purified plasminogen or tPA in combination with antibodies against human laminin-5 (Fig. 8). Laminin-5 antibodies generate staining patterns in circles and arcs at the basal aspect of the cells (Fig. 8, a, d, g, and j). In addition, some staining is also seen in areas of the substrate not covered by cells, as reported previously (Rousselle et al., 1991; Baker et al., 1996a ; Stahl et al., 1997). Interestingly, plasminogen staining in both MCF-10A and pp126 cell cultures colocalizes almost exactly with laminin-5 (Fig. 8, a, b, d, and e). It should be noted that plasminogen associated with pp126 cell matrix is likely derived from the bovine pituitary extract that is added to the serum-free growth medium in which the pp126 cell cultures are maintained. The bovine pituitary extract contains a high level of plasminogen as seen by Western immunoblotting using plasminogen antibody preparations (result not shown).
Figure 8.
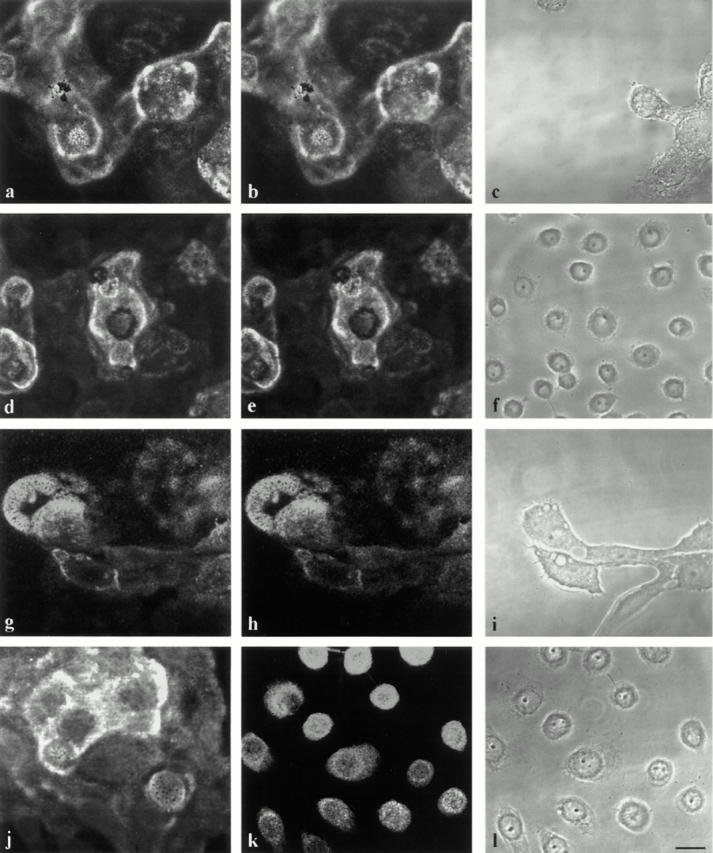
MCF-10A cells (a–c and g–i) and pp126 cells (d–f and j–l) were processed for indirect double-label immunofluorescence microscopy using antibodies against laminin-5 (a, d, g, and j) in combination with either an antibody against plasminogen (b and e) or tPA (h and k). Note that there is colocalization of plasminogen and laminin-5 staining patterns in a and b as well as d and e. In g and h, tPA shows codistribution with laminin-5. In k, tPA localizes to cell bodies but does not colocalize with laminin-5 in j. c, f, i, and l are phase images. Bar, 25 μm.
In MCF-10A cells, a monoclonal antibody against tPA shows an almost identical staining pattern to that generated by an antiserum against laminin-5 (Fig. 8, g and h). In contrast, tPA does not localize to the matrix of pp126 cells, although it shows weak staining of cell bodies (Fig. 8, j and k). The cell bodies of pp126 cells but not matrix are also stained by a uPA antibody, whereas the cells show no staining with an antibody against uPA receptor (result not shown). MCF-10A cells and underlying matrix do not stain positively for uPA or the uPA receptor, suggesting that uPA is not involved in plasmin-mediated laminin-5 processing in vivo, at least in this cell type (result not shown).
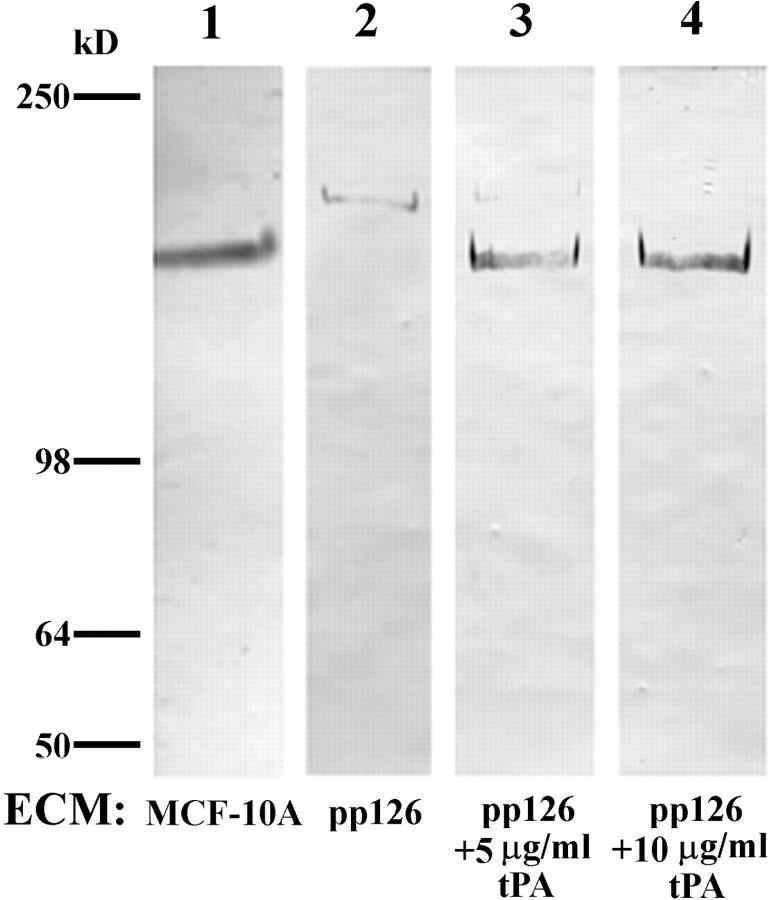
The above results suggest the possibility that lack of tPA in the matrix of pp126 cells may preclude conversion of plasminogen to plasmin and the subsequent plasmin-mediated α3 chain processing in pp126 cell matrix. Therefore, we determined whether addition of purified tPA to pp126 matrix could induce cleavage of the pp126 cell α3 subunit. Western immunoblotting of the tPA-treated pp126 matrix reveals that the laminin-5 α3 subunit is processed to a 160-kD species that comigrates with the laminin-5 α3 subunit in MCF-10A matrix (Fig. 9). In addition, SCC12 cells show less motility on the tPA-treated pp126 matrix (Fig. 4).
Figure 9.
Approximately 10 μg of untreated MCF-10A cell matrix (lane 1), untreated pp126 cell matrix (lane 2), or pp126 cell matrix treated for 16 h with tPA (∼50 μg of matrix incubated for 16 h with 1 ml of tPA at a concentration of either 5 or 10 μg/ml) (lanes 3 and 4) were subjected to SDS-PAGE on 6% gels, transferred to nitrocellulose, and then processed for immunoblotting using the laminin-5 α3 subunit antibody 10B5. In untreated pp126 cell matrix, the α3 subunit shows a molecular weight of 190 kD (lane 2) (refer to Fig. 2). The α3 chain from tPA-treated pp126 matrices comigrates with the α3 chain of MCF-10A matrix at 160 kD (lanes 1, 3, and 4). Note the small amount of residual 190-kD polypeptide in the 5 μg/ml tPA-treated pp126 matrix (lane 3). Molecular weight standards are shown to the left.
In Vitro Interaction.
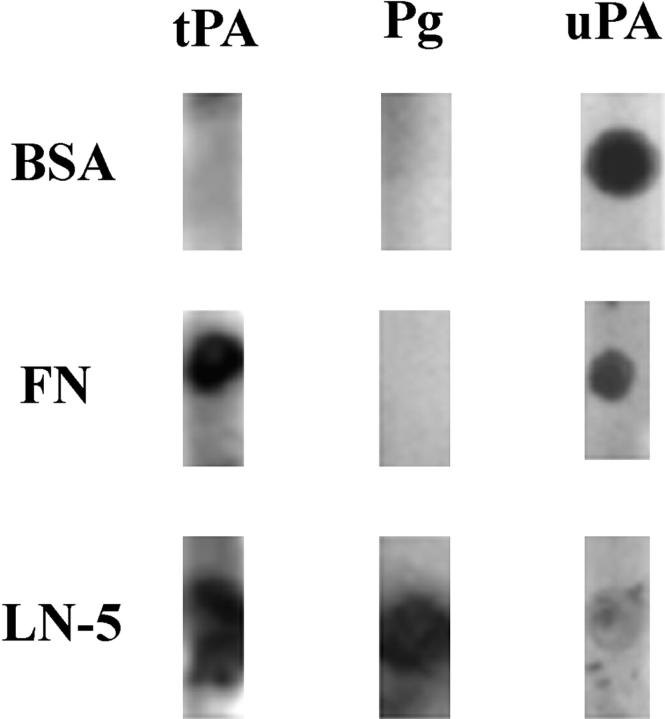
The observed colocalization of tPA and plasminogen with laminin-5 in fixed MCF-10A cell matrix suggests that both might bind directly to laminin-5. This was confirmed using a dot blot overlay assay. Purified human laminin-5 and the control proteins fibronectin and BSA were blotted onto nitrocellulose. Subsequently, tPA, uPA, or plasminogen were incubated in solution with the membranes overnight. The blots were then processed by immunoblotting using antibodies against tPA, plasminogen, or uPA. tPA binds to laminin-5 and to fibronectin (Fig. 10). Plasminogen binds only to laminin-5, whereas uPA binds both BSA and fibronectin (Fig. 10). uPA binds poorly to laminin-5 (Fig. 10).
Figure 10.
Approximately 1 ng of laminin-5 (LN-5), fibronectin (FN), and bovine serum albumin (BSA) were dotted onto nitrocellulose (left). After blocking, the membranes were incubated with 20 μg/ml of tPA, plasminogen (Pg), or uPA overnight at 4°C (top). Bound protein was then detected with the appropriate antibody. Note that tPA binds both fibronectin and laminin-5, whereas plasminogen binds only laminin-5. uPA binds both BSA and fibronectin and also shows some binding to laminin-5.
Discussion
Laminin-5 has been reported to function in the nucleation of hemidesmosome assembly and as an adhesive factor that retards cell motility (Baker et al., 1996a ; O'Toole et al., 1997). In contrast, some authors have provided evidence that laminin-5 enhances cell motility and is expressed at the migrating edges of certain tumor cell populations (Kikkawa et al., 1994; Pyke et al., 1994, 1995; Zhang and Kramer, 1996). The data presented here indicate that posttranslational processing of the α3 subunit of laminin-5 may modulate laminin-5 function. In the case of laminin-5 derived from pp126 cells, the α3 subunit is ∼190-kD, similar to the size of the unprocessed laminin-5 α chain identified by others (Marinkovich et al., 1992; Matsui et al., 1995a ). Laminin-5–rich matrix that contains this unprocessed α3 chain supports keratinocyte cell motility and does not induce hemidesmosome assembly. Upon plasmin-mediated proteolytic cleavage of the α3 subunit to 160 kD, laminin-5–rich matrix becomes competent to trigger the assembly of hemidesmosomes, leading to decreased cell motility. The latter suggestion is in conflict with data presented by Kikkawa (1994), who have shown that buffalo rat liver cells scatter on processed laminin-5. However, one likely explanation for this apparent anomaly is that liver cells do not assemble hemidesmosomes and therefore, may not be capable of establishing stable anchorage sites on laminin-5.
Our initial experiments were performed using the laminin-5–rich matrix of pp126 cells. This matrix contains no detectable β1 laminin subunit suggesting that there is little, if any, laminin-6 in the material that could contribute to the phenomena we detail. However, using this material, we could not rule out the possibility that a minor nonlaminin-5 component of pp126 matrix is involved in the nucleation of hemidesmosome formation. Thus, to confirm a specific role for plasmin-treated pp126 laminin-5 in hemidesmosome assembly, we have made use of a function- inhibiting laminin-5 antibody. Our data reveal that this antibody inhibits the ability of the plasmin-modified pp126 laminin-5 to nucleate hemidesmosome formation in SCC12 cells. Furthermore, as final confirmation of the importance of laminin-5 and its processing in determining its function, we have shown that SCC12 cells migrate less and assemble more hemidesmosomes on plasmin-modified purified laminin-5 compared to untreated purified laminin-5.
Based on the observation that laminin-5 is expressed at the leading edge of migrating tumor cells, one might hypothesize that this laminin-5 includes the 190-kD α3 subunit rather than the 160-kD processed form (Pyke et al., 1994, 1995). Of course, the actively migrating buds of tumors are likely to be rich in a variety of proteinases that could further degrade laminin-5 and impact its function. In this regard, it has been recently shown that the γ2 chain of laminin-5 is proteolyzed to an 80-kD species by MMP-2 and that laminin-5 containing the truncated γ2 chain induces cell motility (Giannelli et al., 1997).
The specific structural and functional impact of plasmin treatment on the α3 chain of pp126 laminin-5 in our in vitro assays led us to investigate the possibility that the plasminogen activator/plasmin system is involved in processing laminin-5 in our cultured cell models. Indeed, our data support the hypothesis that codistribution of enzyme (plasmin) and substrate (laminin-5) facilitates modification of laminin-5 structure with a resulting impact on its function. We have shown that plasminogen is associated with laminin-5 matrix in cultured epithelial cells at the morphological level. Furthermore, tPA is also associated only with the matrix of those cells whose laminin-5 contains a processed α3 chain. tPA is not apparently present in matrix of pp126 cells, in which the α3 chain appears in its unprocessed form. Moreover, we show using a dot blot overlay assay that both plasminogen and tPA can bind laminin-5. Intriguingly, we have been able to induce processing of the α3 chain of pp126 cell laminin-5 as well as conversion from a high to low motility matrix by simply adding tPA to pp126 cell extracellular matrix. Since tPA is a proteinase with extremely limited substrate specificity, it is unlikely that tPA alone could proteolyze the α3 chain (Stack et al., 1995). Together, these data suggest that addition of tPA to the pp126 cells is able to catalyze the conversion of plasminogen to plasmin, which can then target the α3 subunit.
The role of plasmin and tPA in generating a truncated laminin-5 α3 subunit in this system has striking parallels to the relationship of plasmin and tPA to laminin-1. For example, extracellular tPA secreted by B16F10 melanoma cells and human colon carcinoma cells induces hydrolysis of laminin-1 in a plasminogen-dependent manner (Stack et al., 1993; Tran-Thang et al., 1994; Sordat et al., 1995). Both tPA and plasminogen exhibit high-affinity binding to the α1 subunbit of laminin-1 (Moser et al., 1993). Moreover, full-length laminin-1, as well as a short peptide of the laminin-1 α1 subunit containing the sequence SRARKQAASIKVAV, is able to stimulate the tPA-catalyzed activation of plasmin from plasminogen (Stack and Pizzo, 1993; Stack et al., 1994 a). In this regard, the α3 subunit of the laminin-5 isoform contains a similar sequence (IQQARDAASKVAV) just upstream of the putative start of its globular or G domain. As we have previously shown that the G domain of the laminin-5 α3 chain is essential for nucleating hemidesmosomes (Baker et al., 1996a ), tPA/ plasmin-mediated cleavage of laminin-5 may generate a truncated, functional G domain that is capable of triggering hemidesmosome assembly. This possibility is supported by our finding that plasmin cleavage occurs at the COOH terminus. Furthermore, a stimulatory effect of laminin-5 on tPA-induced plasminogen activation, similar to that observed with laminin-1, would support a feedback mechanism, whereby epithelial cells indirectly influence their own behavior by affecting the structure and function of their own matrix molecules in their extracellular environment (Roskelly et al., 1995).
Based on our results, we propose the following model for nucleation of hemidesmosomes in cultured epithelial cells. Laminin-5, containing a 190-kD α3 subunit, is secreted into the extracellular environment by epithelial cells. Plasminogen associates directly with the matrix by binding laminin-5. Only certain epithelial cell types such as MCF-10A secrete tPA which, like plasminogen, also associates with laminin-5. The spatial colocalization of plasminogen and tPA on the laminin-5 molecule allows for efficient production of plasmin from plasminogen, catalyzed by tPA. The newly generated plasmin then initiates cleavage of the α3 subunit of laminin-5. After cleavage of its α3 subunit, laminin-5 is then able organize the appropriate integrins and other cell surface-associated proteins to nucleate assembly of a hemidesmosome (Baker et al., 1996a ). Those cell types, such as pp126 cells that do not express tPA in their extruded matrix, are thus incapable of forming hemidesmosomes because the α3 chain of the laminin-5 molecule is incompletely processed.
In summary, we have elucidated a regulatory enzymatic cascade which, in cells secreting the appropriate enzymes, appears to result in proteolysis of laminin-5 and subsequent nucleation of hemidesmosome assembly. Many investigators have recognized the ability of extracellular matrix to alter cellular behavior. Our data, showing the downstream effects of secretion of specific enzymes that bind to and modify laminin-5, indicate that epithelial cells indirectly regulate their own behavior, via changes in the surrounding matrix.
Acknowledgments
We are grateful to M. Ravosa for performing the statistical analyses of our motility assay data and to T.-L. Chen for help in using the video microscope. We thank X. He for technical assistance (all three from Northwestern University Medical School, Chicago, IL).
This work is supported by National Institutes of Health grants to J.C.R. Jones (GM38470 and PO1 DE12328) and M.S. Stack (CA58900). L.E. Goldfinger is supported by training grant (T32 CA09560) from the National Cancer Institute.
Abbreviations used in this paper
- ECM
extracellular matrix
- His
histidine
- MMP
matrix metalloproteinase
- NHEK
normal human keratinocyte
- SSC
squamous cell carcinoma
- tPA
tissue-type plasminogen activator
- uPA
urinary-type plasminogen activator
Footnotes
Address all correspondence to Jonathan C.R. Jones, Department of Cell and Molecular Biology, Morton 4-616, Northwestern University School of Medicine, 303 East Chicago Avenue, Chicago, IL 60611. Tel.: (312) 503-1412. Fax: (312) 503-6475. E-mail: j-jones3@nwu.edu
References
- Baker SE, Hopkinson SB, Fitchmun M, Andreason GL, Frasier F, Plopper G, Quaranta V, Jones JCR. Laminin-5 and hemidesmosomes: role of the α3 subunit in hemidesmosome stability and assembly. J Cell Sci. 1996a;109:2509–2520. doi: 10.1242/jcs.109.10.2509. [DOI] [PubMed] [Google Scholar]
- Baker SE, DiPasquale A, Stock EL, Plopper G, Quaranta V, Fitchmun M, Jones JCR. Morphogenetic effects and utility in organ culture of a soluble laminin variant, laminin-5. Exp Cell Res. 1996b;228:262–270. doi: 10.1006/excr.1996.0325. [DOI] [PubMed] [Google Scholar]
- Borradori L, Sonnenberg A. Hemidesmosomes: role in adhesion, signaling and human diseases. Curr Opin Cell Biol. 1996;8:647–656. doi: 10.1016/s0955-0674(96)80106-2. [DOI] [PubMed] [Google Scholar]
- Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin α3β1 in epithelial basement membranes. Cell. 1991;65:599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- Champliaud MF, Lunstrum GP, Rousselle P, Nishiyama T, Keene DR, Burgeson RE. Human amnion contains a novel laminin variant, laminin-7, which like laminin-6, covalently associates with laminin-5 to promote stable epithelial-stromal attachment. J Cell Biol. 1997;132:1189–1198. doi: 10.1083/jcb.132.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghannam, A., L. Starr, and J.C.R. Jones. 1998. Laminin-5 coating enhances epithelial cell attachment, spreading and hemidesmosome assembly on Ti-6Al-4V implant material in vitro. J. Biomed. Mater. Res. In press. [DOI] [PubMed]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz, D. 1984. In Methods for Preparation of Media Supplements and Substrata. Vol. 1. D.W. Barnes, D.A. Sirbasku, and G.H. Stao, editors. Alan R. Liss, New York. 275–293.
- Green KJ, Jones JCR. Desmosomes and hemidesmosomes: structure and function of molecular components. FASEB (Fed Am Soc Exp Biol) J. 1996;10:871–880. doi: 10.1096/fasebj.10.8.8666164. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and D. Lane. 1988. In Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 92–121.
- Hormia M, Falk-Marzillier J, Plopper G, Tamura RN, Jones JCR, Quaranta V. Rapid spreading and mature hemidesmosome formation in HaCaT keratinocytes induced by incubation with soluble laminin-5r. J Invest Dermatol. 1995;105:557–561. doi: 10.1111/1523-1747.ep12323451. [DOI] [PubMed] [Google Scholar]
- Jones JCR, Asmuth J, Baker SE, Langhofer M, Roth SI, Hopkinson SB. Hemidesmosomes: extracellular matrix/intermediate filament connectors. Exp Cell Res. 1994;213:1–11. doi: 10.1006/excr.1994.1166. [DOI] [PubMed] [Google Scholar]
- Kallunki P, Sainio K, Eddy R, Byers M, Kallunki T, Sariola H, Beck K, Hirvonen H, Shows TB, Tryggvason K. A truncated laminin chain homologous to the B2 chain: structure, spatial expression, and chromosomal assignment. J Cell Biol. 1992;119:679–693. doi: 10.1083/jcb.119.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa Y, Umeda M, Miyazaki K. Marked stimulation of cell adhesion and motility by ladsin, a laminin-like scatter factor. J Biochem (Tokyo) 1994;116:862–869. doi: 10.1093/oxfordjournals.jbchem.a124608. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langhofer M, Hopkinson SB, Jones JCR. The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosome assembly in vitro. J Cell Sci. 1993;105:753–764. doi: 10.1242/jcs.105.3.753. [DOI] [PubMed] [Google Scholar]
- Little SS, Johnson DA. Human mast cell tryptase isoforms: separation and examination of substrate-specificity differences. Biochem J. 1995;307:341–346. doi: 10.1042/bj3070341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinda KM, Kleinman HK. The laminins. Int J Biochem Cell Biol. 1996;28:957–959. doi: 10.1016/1357-2725(96)00042-8. [DOI] [PubMed] [Google Scholar]
- Marinkovich MP, Lunstrum GP, Burgeson RE. The anchoring filament protein kalinin is synthesized and secreted as a high molecular weight precursor. J Biol Chem. 1992;267:17900–17906. [PubMed] [Google Scholar]
- Matsui C, Wang CK, Nelson CF, Bauer EA, Hoeffler WK. The assembly of laminin-5 subunits. J Biol Chem. 1995a;270:23496–23503. doi: 10.1074/jbc.270.40.23496. [DOI] [PubMed] [Google Scholar]
- Matsui C, Nelson CF, Hernandez GT, Herron GS, Bauer EA, Hoeffler WK. γ2 chain of laminin-5 is recognized by monoclonal antibody GB3. J Invest Dermatol. 1995b;105:648–652. doi: 10.1111/1523-1747.ep12324108. [DOI] [PubMed] [Google Scholar]
- Moser TL, Enghild JJ, Pizzo SV, Stack MS. The extracellular matrix proteins laminin and fibronectin contain binding domains for human plasminogen and tissue plasminogen activator. J Biol Chem. 1993;268:18917–18923. [PubMed] [Google Scholar]
- Niessen CM, Hogervorst F, Jaspars LH, De Melker AA, Delwel GO, Hulsman EHM, Kuikman I, Sonnenberg A. The α6β4 integrin is a receptor of both laminin and kalinin. Exp Cell Res. 1994;211:360–367. doi: 10.1006/excr.1994.1099. [DOI] [PubMed] [Google Scholar]
- Oda D, Bigler L, Lee P, Blanton R. HPV immortalization of human oral epithelial cells: a model for carcinogenesis. Exp Cell Res. 1996;226:164–169. doi: 10.1006/excr.1996.0215. [DOI] [PubMed] [Google Scholar]
- O'Toole EA, Marinkovich MP, Hoeffler W, Furthmayr H, Woodley DT. Laminin-5 inhibits human keratinocyte migration. Exp Cell Res. 1997;233:330–339. doi: 10.1006/excr.1997.3586. [DOI] [PubMed] [Google Scholar]
- Pyke C, Romer J, Kallunki P, Lund LR, Ralfkiaer E, Dano K, Tryggvason K. The gamma 2 chain of kalinin/laminin-5 is preferentially expressed in invading malignant cells in human cancers. Am J Pathol. 1994;145:782–791. [PMC free article] [PubMed] [Google Scholar]
- Pyke C, Salo S, Ralfkiaer E, Romer J, Dano K, Tryggvason K. Laminin-5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase activator in budding cancer cells in colon adenocarcinomas. Cancer Res. 1995;55:4132–4139. [PubMed] [Google Scholar]
- Ranby M. Studies on the kinetics of plasminogen activation by tissue plasminogen activator. Biochim Biophys Acta. 1982;704:461–469. doi: 10.1016/0167-4838(82)90068-1. [DOI] [PubMed] [Google Scholar]
- Riddelle KS, Green KJ, Jones JCR. Formation of hemidesmosomes in vitro by a transformed rat bladder cell. J Cell Biol. 1991;112:159–168. doi: 10.1083/jcb.112.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskelly CD, Srebow A, Bissell MJ. A hierarchy of ECM-mediated signaling regulates tissue-specific gene expression. Curr Opin Cell Biol. 1995;7:736–747. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114:567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MC, Tizard R, VanDevanter DR, Carter WG. Cloning of the LamA3 gene encoding the α3 chain of the adhesive ligand epiligrin. J Biol Chem. 1994;269:22779–22787. [PubMed] [Google Scholar]
- Sordat BC, Tran-Thang C. Laminin degradation by human colon carcinoma cells: a role for urinary and tissue plasminogen activators. Invasion Metastasis. 1995;14:223–233. [PubMed] [Google Scholar]
- Stack MS, Pizzo SV. Modulation of tissue plasminogen activator-catalyzed plasminogen activation by synthetic peptides derived from the amino-terminal heparin binding domain of fibronectin. J Biol Chem. 1993;268:18924–18928. [PubMed] [Google Scholar]
- Stack MS, Pizzo SV. The effect of substituted laminin A chain- derived peptides on the conformation and activation kinetics of plasminogen. Arch Bioch Biophys. 1994;309:117–122. doi: 10.1006/abbi.1994.1093. [DOI] [PubMed] [Google Scholar]
- Stack MS, Gray RD, Pizzo SV. Modulation of murine B16F10 melanoma plasminogen activator production by a synthetic peptide derived from the laminin A chain. Cancer Res. 1993;53:1998–2004. [PubMed] [Google Scholar]
- Stack MS, Rinehart AR, Pizzo SV. Comparison of plasminogen binding and activation on extracellular matrices produced by vascular smooth muscle and endothelial cells. Eur J Biochem. 1994;226:937–943. doi: 10.1111/j.1432-1033.1994.00937.x. [DOI] [PubMed] [Google Scholar]
- Stack, M.S., E.L. Madison, and S.V. Pizzo. 1995. In Molecular Basis of Thrombosis and Hemostasis. K.A. High and H.R. Roberts, editors. Marcel Decker, Inc., New York. 479–494.
- Stahl S, Weitzman S, Jones JCR. The role of laminin-5 and its receptors in mammary epithelial cell branching morphogenesis. J Cell Sci. 1997;110:55–63. doi: 10.1242/jcs.110.1.55. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Spurr-Michaud S, Tisdale A, Elwell J, Gipson IK. α6β4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci USA. 1990;87:8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz APN, Roskelly C, Bissell MJ. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura RN, Oda D, Quaranta V, Plopper G, Lambert R, Glaser S, Jones JCR. Coating of titanium alloy with soluble laminin-5 promotes cell attachment and hemidesmosome assembly in gingival epithelial cells: potential application to dental implants. J Periodontal Res. 1997;32:287–294. doi: 10.1111/j.1600-0765.1997.tb00536.x. [DOI] [PubMed] [Google Scholar]
- Timpl R, Brown JC. The laminins. Matrix Biol. 1994;14:275–281. doi: 10.1016/0945-053x(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Tran-Thang C, Voullamoz D, Kruithof EK, Sordat B. Human Co115 colon carcinoma cells potentiate degradation of laminin mediated by tissue-type plasminogen activator. J Cell Phys. 1994;161:285–292. doi: 10.1002/jcp.1041610213. [DOI] [PubMed] [Google Scholar]
- Tryggvason K. The laminin family. Curr Opin Cell Biol. 1993;5:877–882. doi: 10.1016/0955-0674(93)90038-r. [DOI] [PubMed] [Google Scholar]
- Vailly J, Verrando P, Champliaud MF, Gerecke D, Wagman DW, Baudoin C, Aberdam D, Burgeson R, Bauer E, Ortonne JP. The 100-kDa chain of nicein/kalinin is a laminin B2 chain variant. Eur J Biochem. 1994;219:209–218. doi: 10.1111/j.1432-1033.1994.tb19932.x. [DOI] [PubMed] [Google Scholar]
- Verrando P, Hsi B-L, Yeh C-J, Pisani A, Serieys N, Ortonne J-P. Monoclonal antibody GB3, a new probe for the study of human basement membranes and hemidesmosomes. Exp Cell Res. 1987;170:116–128. doi: 10.1016/0014-4827(87)90121-2. [DOI] [PubMed] [Google Scholar]
- Wun TC. Plasminogen activation: biochemistry, physiology, and therapeutics. Crit Rev Biotech. 1988;8:131–148. doi: 10.3109/07388558809150542. [DOI] [PubMed] [Google Scholar]
- Young TN, Rodriguez GC, Rinehart AR, Bast RC, Jr, Pizzo SV, Stack MS. Characterization of gelatinases linked to extracellular matrix invasion in ovarian adenocarcinoma: purification of matrix metalloproteinase 2. Gynecol Oncol. 1996;62:89–99. doi: 10.1006/gyno.1996.0195. [DOI] [PubMed] [Google Scholar]
- Zackroff RV, Goldman AE, Jones JCR, Steinert PM, Goldman RD. Isolation and characterization of keratin-like proteins from cultured cells with fibroblastic morphology. J Cell Biol. 1984;98:1231–1237. doi: 10.1083/jcb.98.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kramer R. Laminin-5 deposition promotes keratinocyte motility. Exp Cell Res. 1996;227:309–333. doi: 10.1006/excr.1996.0280. [DOI] [PubMed] [Google Scholar]