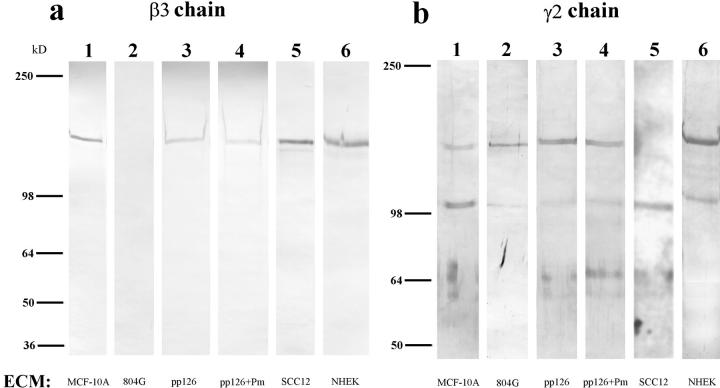
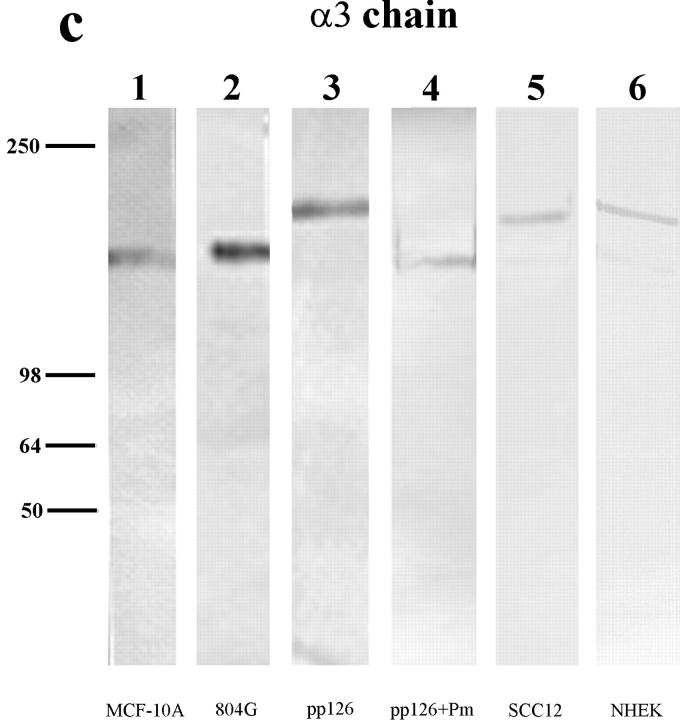
Figure 2.
The laminin-5 subunit compositions of the extracellular matrices (ECM) of MCF-10A, 804G, pp126, SCC12, and NHEK cells are shown (lanes 1–6). Approximately 10 μg of matrix protein was run on each lane of a 6% gel, transferred to nitrocellulose, and then processed for immunoblotting using laminin-5 subunit– specific antibody preparations. (a) The laminin-5 β3 chain was identified using the monoclonal antibody clone 17. Clone 17 antibody recognizes a protein of 145 kD in all of the human matrices but shows no reactivity with 804G cell matrix (compare lane 2 with lanes 1, 3, 5, and 6). (b) Antiserum J20, against the laminin-5 γ2 chain, recognizes 155-kD polypeptides in matrix preparations derived from MCF-10A, 804G, pp126, and NHEK cells (lanes 1, 2, 3, and 6), as well as a 105-kD species in the matrices of MCF-10A, 804G, SCC12, and NHEK cells (lanes 1, 2, 5, and 6). (c) The laminin-5 α3 chain is identified using the mouse monoclonal antibody 10B5, which recognizes a polypeptide migrating at 160 kD in 804G and MCF-10A cell matrix (lanes 1 and 2), and a protein of 190 kD in the matrices of pp126, NHEK, and SCC12 cells (lanes 3, 5, and 6).Approximately 50 μg of the matrix of pp126 cells was treated for 90 min with a 1-ml PBS solution containing plasmin (+Pm) at a concentration of 1 μg/ml and then the treated matrix preparation was probed with the β3, γ2, and α3 subunit-specific antibodies (lane 4 in each blot). The mobility of the β3 and γ2 subunits are unaffected by such treatment (compare b, lanes 3 and 4), whereas the α3 subunit migrates at 160 kD, compared with 190 kD in the untreated matrix (compare c, lanes 3 and 4). Molecular weight standards are indicated to the left.