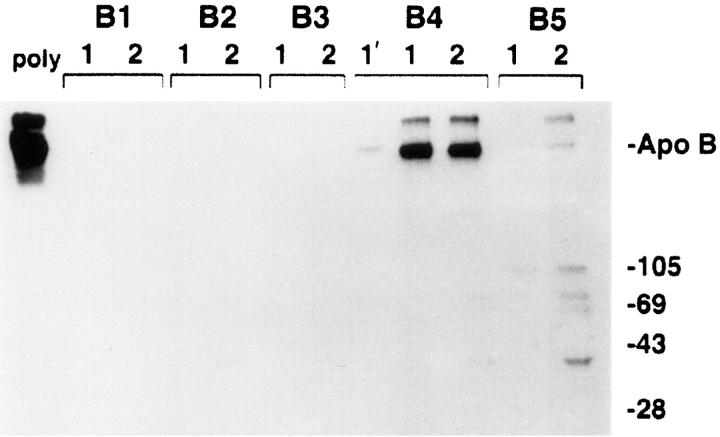
Figure 2.
Comparison of anti-apoB peptide antibodies to the conventional polyclonal anti-LDL in ability to immunoprecipitate apoB. Five hydrophilic peptides of apoB (B1–B5) were synthesized as described in Materials and Methods. Polyclonal antisera to these peptides were raised in rabbits and evaluated by immunoprecipitation of [3H]leucine-labeled HepG2 proteins. [3H]apoB from 500 μl of HepG2 lysis buffer cell extracts was immunoprecipitated with 1 μl of sheep polyclonal anti-human LDL (poly, leftmost lane) or with 20 μl of each antipeptide antisera under normal immunoprecipitation conditions (lanes designated with a 1), or when the immunoprecipitation binding and washing buffers contained final concentrations of 2 mM dithiothreitol and 0.5% deoxycholic acid (lanes designated with a 2). In the lane marked 1′, apoB was immunoprecipitated with 5 μl of the B4 antiserum under normal immunoprecipitation conditions. ApoB was immunoprecipitated and analyzed by SDS-PAGE (3–15% gel) and fluorography. Markers show the location of Coomassie-stained standard apoB (isolated from human plasma) and molecular weight standards (kD). Antiserum from the rabbit injected with the B4 peptide was affinity-purified on an aminolink-B4 peptide affinity column before use in subsequent immunocytochemistry studies.