Figure 2.
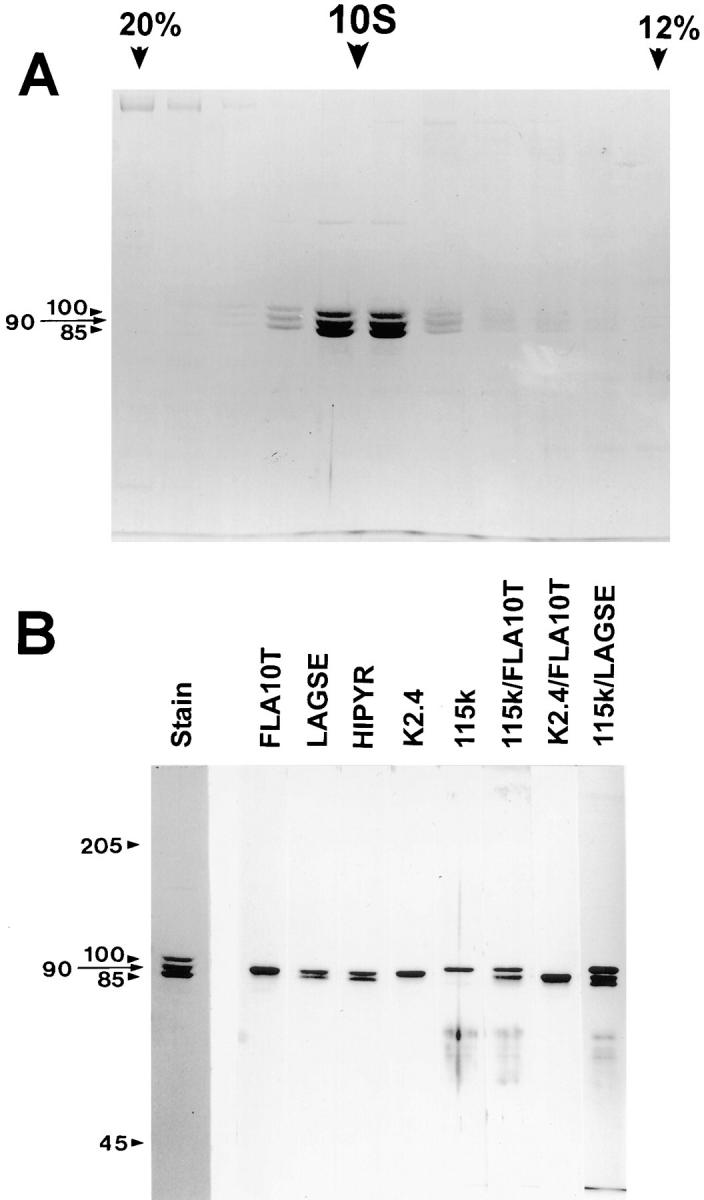
Sucrose density gradient profile of FLA10 kinesin-II and subsequent antibody analysis. The peak FLA10-containing fractions from S400 chromatography were pooled, concentrated, and further fractionated on a 5-ml, 5–20% sucrose gradient. (A) Coomassie-stained gel shows cosedimentation at 9.7 S of three polypeptides at 100, 90, and 85 kD with molar ratios of 0.8, 1.0, and 1.0, respectively. Only the portion of the gradient from 20% (left) to 12% (right) sucrose is shown. (B) Analysis of FLA10 kinesin-II subunits with antibodies. The left panel is a Coomassie-stained gel of sucrose density gradient–purified FLA10 kinesin-II. The right panel contains separate strips from corresponding immunoblots that have been probed with the antibodies listed above each strip. Both of the pan-kinesin peptide antibodies, LAGSE and HIPYR, reacted with both the 85- and 90-kD polypeptides, indicating a high probability that both subunits are kinesin-like proteins. Anti-FLA10T and K2.4, an mAb raised against the 85-kD subunit of sea urchin kinesin-II, reacted only with the 90-kD subunit (FLA10). The polyclonal anti-115k antisera, raised against the 115-kD nonmotor subunit of sea urchin kinesin-II, reacted only with the 100-kD subunit. The identities of the reactive bands were confirmed in lanes probed with mixtures of the antibodies. The mixture of K2.4 and anti-FLA10T reacted only with the 90-kD band, verifying that a monoclonal raised against the smaller of the two kinesin-like subunits (85 kD) of sea urchin kinesin-II recognizes the larger of the two kinesin-like subunits (90 kD) of Chlamydomonas kinesin-II.
