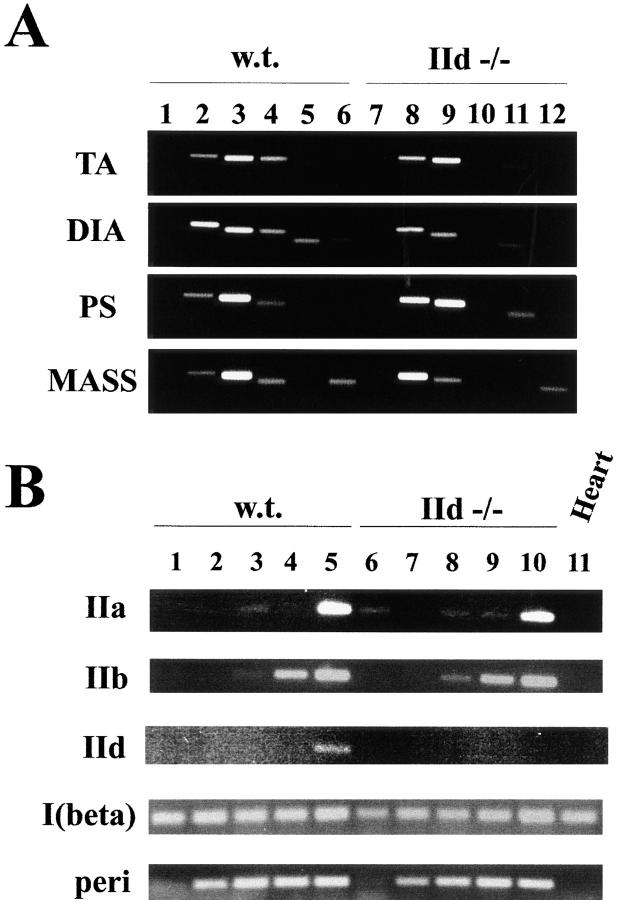
Figure 6.
MyHC gene expression in wild-type and IId null mice. RT reactions were performed as described in Materials and Methods. Oligonucleotide primers for PCR were designed in the 3′-UTRs of MyHC genes. PCR amplification of cDNA was performed as described at 25 cycles for all reactions except MyHC-IId at developmental time points (B), which was performed at 30 cycles. One quarter of each reaction was separated on a 2% agarose gel. (A) RNA was prepared from TA, diaphragm, psoas, and masseter muscles of wild-type (lanes 1–6) and IId null (lanes 7–12) female mice (8 wk old). In lanes 1 and 7, PCR was performed in the presence of all sets of MyHC-specific primers with extracts from control reactions in which RT was not added. PCR reactions were specific for MyHC-IIa, lanes 2 and 8; MyHC-IIb, lanes 3 and 9; MyHC-IId, lanes 4 and 10; MyHC-I (β, slow), lanes 5 and 11; and MyHC-perinatal, lanes 6 and 12. (B) RNA was prepared from wild-type (lanes 1–5) and IId null (lanes 6–10) mice at 12.5 dpc (lanes 1 and 6) and 15.5 dpc (lanes 2 and 7) and at 1 (lanes 3 and 8), 3 (lanes 4 and 9), and 5 d (lanes 5 and 10) after birth. At 12.5 and 15.5 dpc, RNA was prepared from whole embryos. At 1, 3, and 5 d after birth, RNA was prepared from hindlimbs. RNA from adult mouse heart (6 wk) was used as a control in lane 11. PCR reactions contained primers specific for MyHC-IIa, -IIb, -IId, -I (β, slow), and perinatal as indicated.