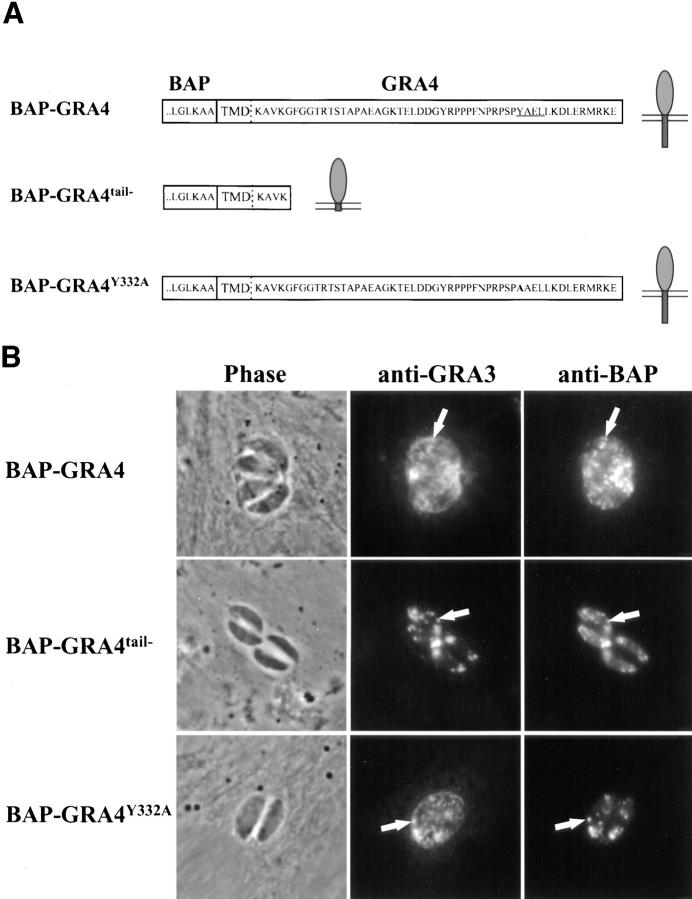
Figure 5.
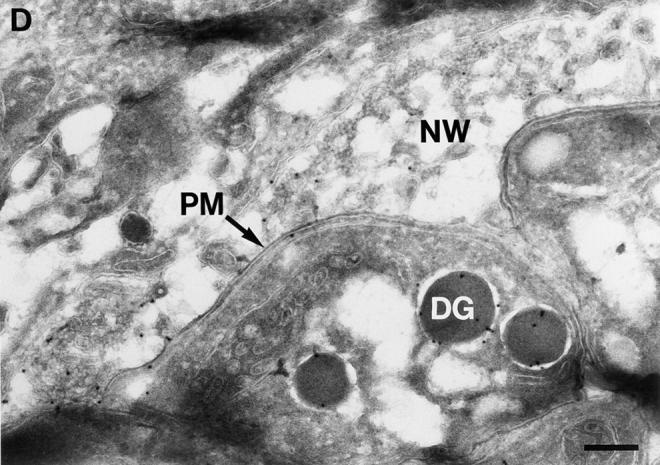
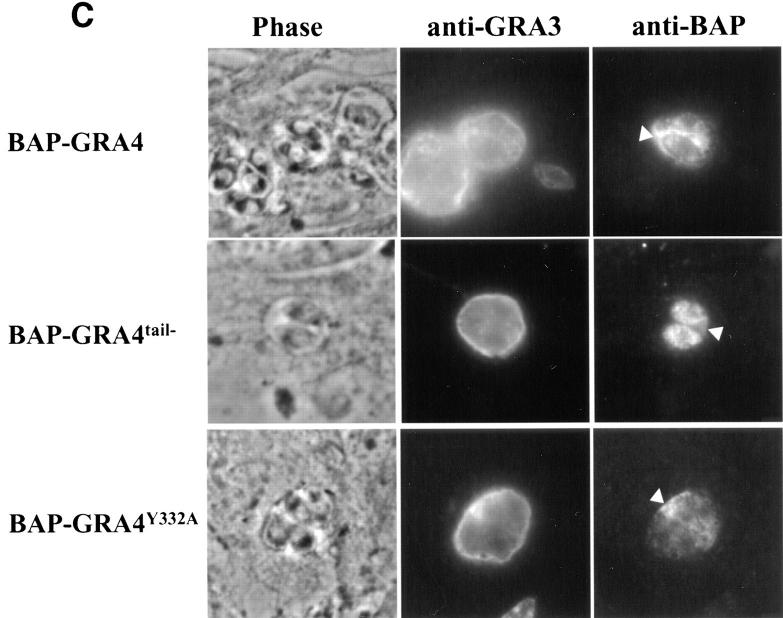
BAP-GRA4 localizes to dense granules and requires the cytoplasmic tail for efficient secretion into the intravacuolar network. (A) Design of BAP-GRA4, BAP-GRA4tail−, and BAP-GRA4Y332A constructs, showing addition of the GRA4 transmembrane and cytoplasmic tail to BAP and the two alterations within the tail. All of the constructs behaved as integral membrane proteins when analyzed in a heterologous in vitro translation/translocation system (not shown). (B) Infected cells containing parasites expressing BAP-GRA4, BAP-GRA4tail−, or BAP-GRA4Y332A were fixed with PFA and permeabilized with Triton X-100 to optimize staining of dense granules and other internal organelles of the parasite. Cells were stained with antibodies to BAP or the dense granule marker GRA3. The BAP signal colocalized with staining for GRA3 in all three chimeras, demonstrating delivery of BAP-GRA4, BAP-GRA4tail−, and BAP-GRA4Y332A to dense granules (arrows). (C) Infected cells containing parasites expressing BAP-GRA4, BAP-GRA4tail−, or BAP-GRA4Y332A were fixed with MeOH to optimize detection of proteins secreted into the vacuolar space. Cells were stained with antibodies to BAP or GRA3. The arrowheads show network staining for the BAP-GRA4 and BAP-GRA4Y332A constructs. Only minimal amounts (arrowhead) of GRA4tail− were detected in the vacuolar space. (D) Cryosections of BAP-GRA4–expressing parasites stained with antibodies to BAP. Gold particles localize primarily to dense granules (DG) and the intravacuolar network (NW), with some staining of the plasma membrane (PM). Bar, 200 nm.