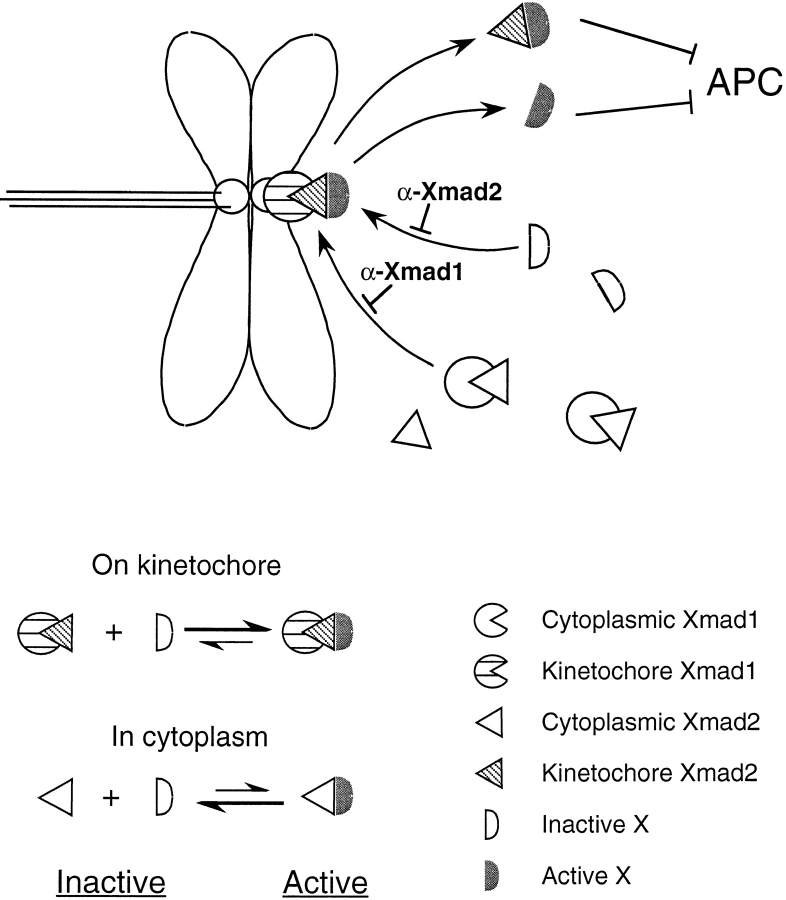
Figure 11.
A model for how Xmad1 and Xmad2 work to activate the spindle checkpoint. Binding of Xmad1 to unattached kinetochores enables its associated Xmad2 to interact with a downstream checkpoint component X. This interaction converts X into a form that is capable of directly or indirectly inhibiting the anaphase-promoting complex (APC). The interaction between Xmad2 and X is unstable when Xmad2 is not associated with kinetochores, so that the checkpoint is not activated without unattached kinetochores. Increasing the global Xmad2 concentration drives the complex formation by mass action even in the absence of kinetochores. At a high enough concentration of Xmad2, the level of Xmad2–X complex becomes comparable to that induced through kinetochore-associated Xmad2 and results in constitutive activation of the spindle checkpoint. A likely candidate for X is Cdc20.