Figure 6.

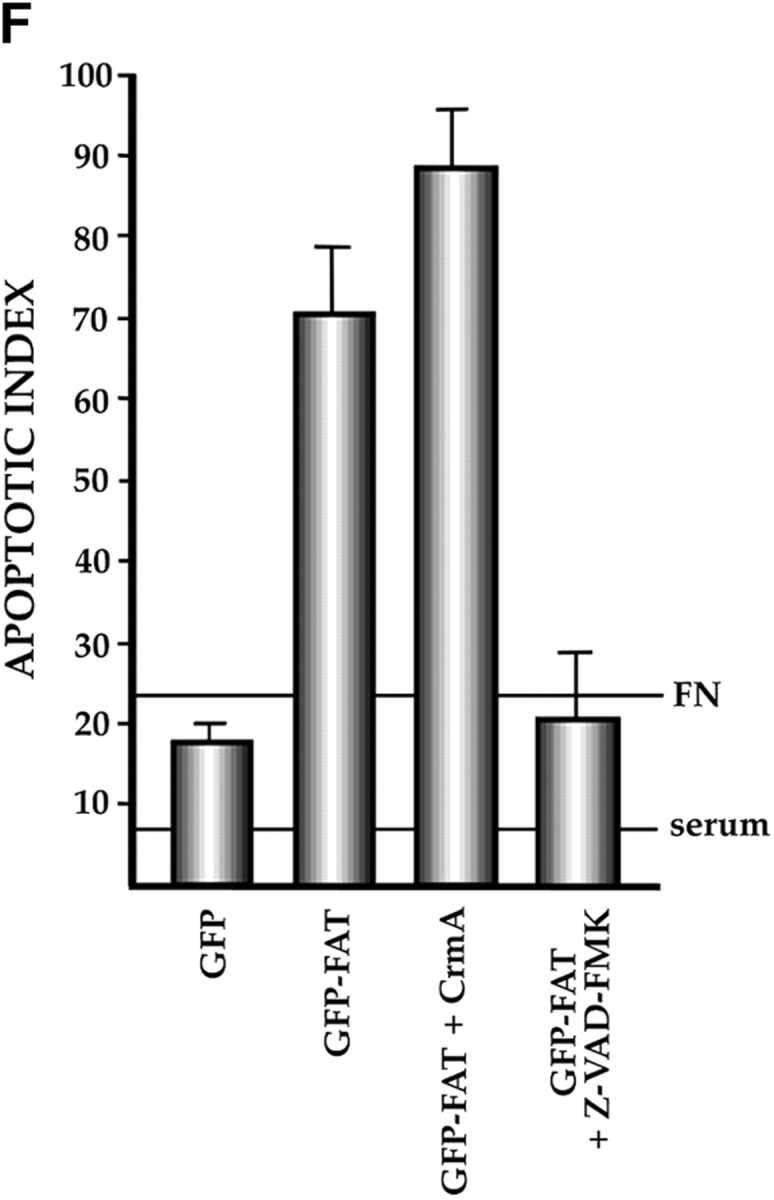
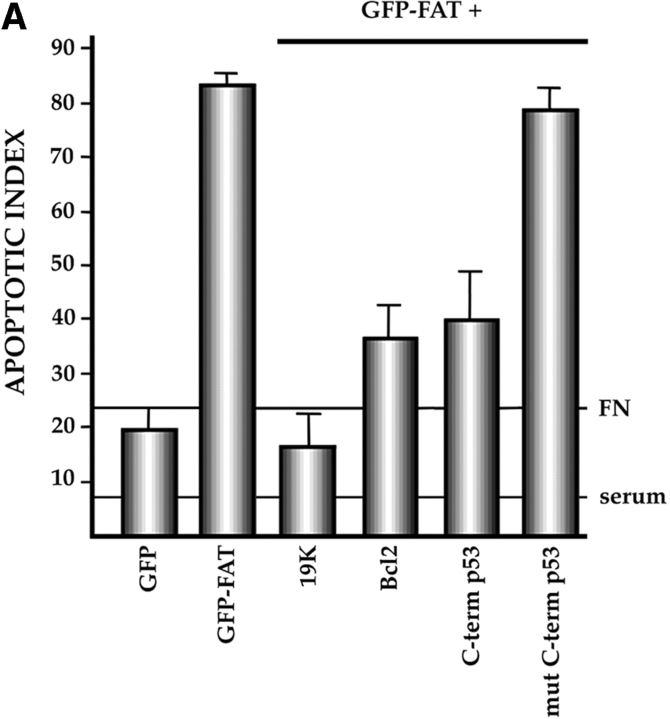
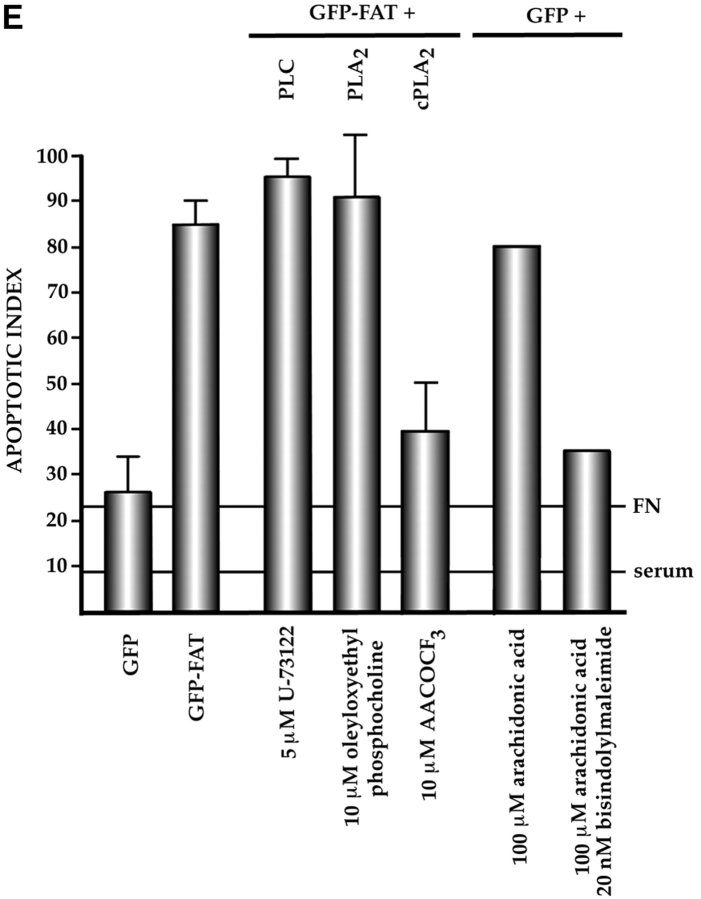
Characterization of the apoptotic pathway triggered in the absence of ECM survival signals in serum-deprived RSF: pharmacological and dominant-negative strategies reveal an apoptotic pathway that is regulated by p53, activated by cPLA2 and PKC λ/ι, and resistant to CrmA. (A) Blocking the function of p53 permits survival of primary RSF despite suppression of FAK function by GFP–FAT. GFP–FAT was cotransfected into fibroblasts plated on fibronectin, with or without either E1B 19K, Bcl2, or a GSE corresponding to the COOH-terminal domain of p53 (C-term p53), which inactivates p53 function (see Materials and Methods). The apoptotic index was high in GFP–FAT–transfected cells, but greatly reduced in cells cotransfected with GFP–FAT and one of the following: E1B 19K, Bcl2, or C-term p53. However, cotransfection of C-term p53, which contained mutated putative PKC phosphorylation sites (mut C-term p53), with GFP–FAT did not rescue cells from apoptosis. (B–D) Apoptosis triggered by inactivation of FAK function in serum-deprived anchorage-dependent RSF requires PKC λ/ι. (B) Inhibitors that block function of all isoforms of PKC (bisindolylmaleimide I and chelerythrine chloride) suppressed GFP–FAT–induced apoptosis, whereas calphostin C, which blocks only the PMA-sensitive isoforms (and therefore not PKC λ/ι), did not. (C) RSF express three isotypes of PKC. To detect PKC isotypes, cells were lysed using RIPA buffer in the presence of proteinase inhibitors. PKCs were immunoprecipitated and detected with anti-PKC α, β, γ, δ, ε, ζ, o, ι, λ, and μ antibodies (Transduction Laboratories, Lexington, KY) after separation in 10% SDS–polyacrylamide gels. Only PKC ε, λ, and ι were detected. + control, PKC isoform standard; − control, precipitation of RSF lysate with nonimmune IgG; anti-PKC, precipitation with antibodies specific for the PKC isoforms. (D). Coexpression of dominant-negative (DN) PKC λ/ι, but not DN PKC ε, blocked GFP–FAT–triggered apoptosis. Cotransfection of the wild-type PKC isoforms with GFP–FAT did not rescue cells from apoptosis. Transfection of wild-type isoforms into GFP-transfected cells did not promote apoptosis. (E) Apoptosis triggered by inactivation of FAK function in serum-deprived anchorage-dependent RSF requires cPLA2. Arachidonic acid induced apoptosis in nontransfected or GFP-transfected RSF. Phospholipases catalyze the release of arachidonic acid from phospholipids. AACOCF3, an inhibitor of cPLA2, but not inhibitors of secretory and Ca2+-independent PLA2 or inhibitors of PLC, rescued cells from apoptosis triggered by GFP–FAT. The panPKC inhibitor bisindolylmaleimide I blocked apoptosis triggered by arachidonic acid, suggesting that cPLA2 is upstream of PKC λ/ι in this pathway. (F) Effects of blocking large and small prodomain caspase functions on apoptosis in GFP–FAT–transfected primary RSF. RSF were cotransfected with GFP–FAT and CrmA or with GFP–FAT alone. At 16 h, the apoptotic index of RSF transfected with GFP–FAT was high whether or not CrmA was also transfected. The small prodomain caspase inhibitor, Z-VAD-FMK, inhibited formation of condensed and fragmented nuclei in serum-deprived GFP–FAT–transfected fibroblasts plated on fibronectin.