Figure 6.
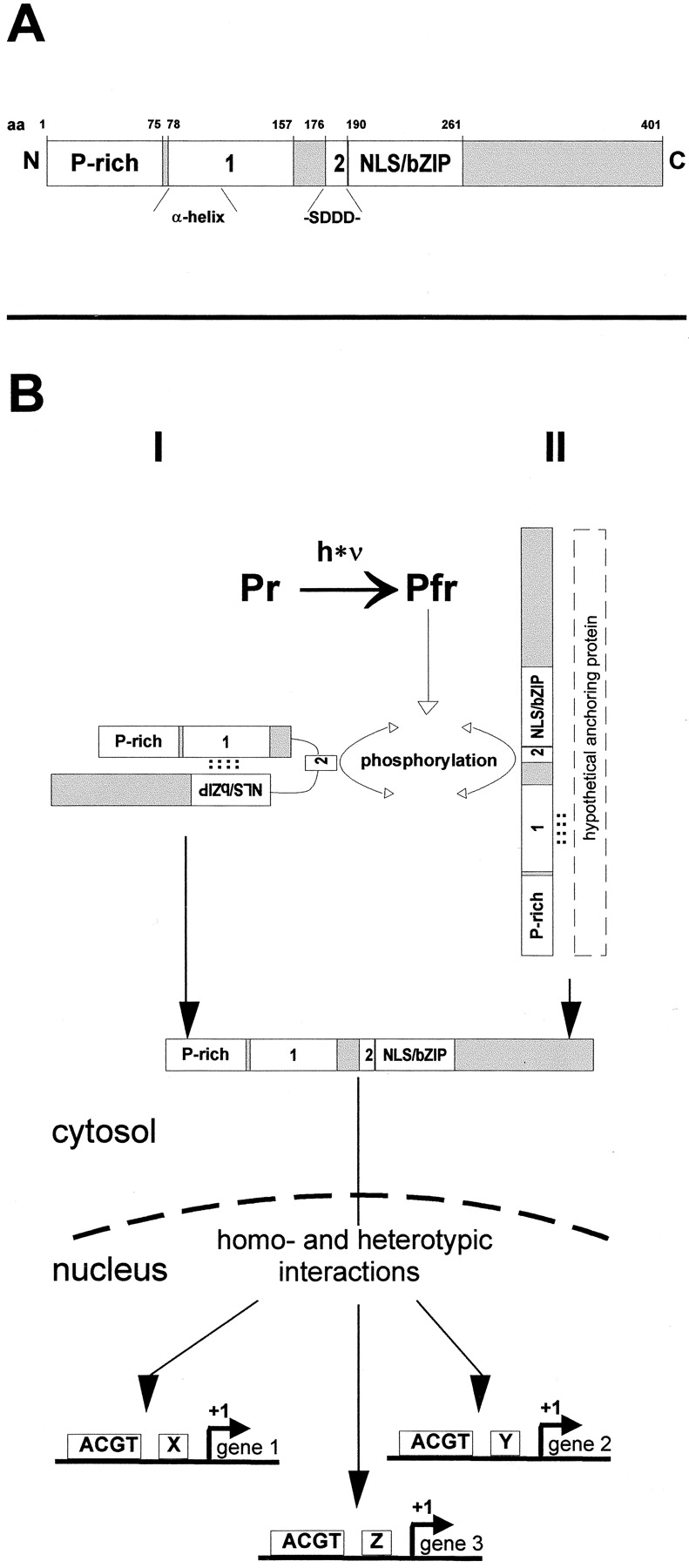
(A) Schematic representation of functional domains within CPRF2. P-rich, prolin-rich transactivation domain (Rügner, A., and E. Schäfer, unpublished data). 1, retention domain 1 that has strong homology to the α-helical cytoplasmic retention domain (α-helix) of the mammalian heat shock factor 2. 2, acidic retention domain 2 that contains a CKII phosphorylation site as indicated (-SDDD-). NLS/bZIP, nuclear localization sequence-containing basic-region leucine-zipper domain. N, NH2 terminus; C, COOH terminus; aa, amino acid. (B) Alternative models of phy-regulated nuclear import of CPRF2 in parsley cells. Pfr formation induces phosphorylation of the CKII site within retention domain 2 of CPRF2. This modification either abolishes intracellular masking of the NLS (model I) or results in the release of CPRF2 from a cytoplasmic anchoring protein (model II). In both cases, accession to the NLS results in nuclear import, homo- and/or heterotypic CPRF2–protein interactions, and binding of CPRF2-containing transcription factor complexes to promoters of light-regulated genes. Further explanation in the text. Pr, inactive form of phytochrome; Pfr, active form of phytochrome; ACGT, G-box like elements; x, y, z, cis-elements other than G-box like motifs; see A for other abbreviations and notes.
