Figure 2.
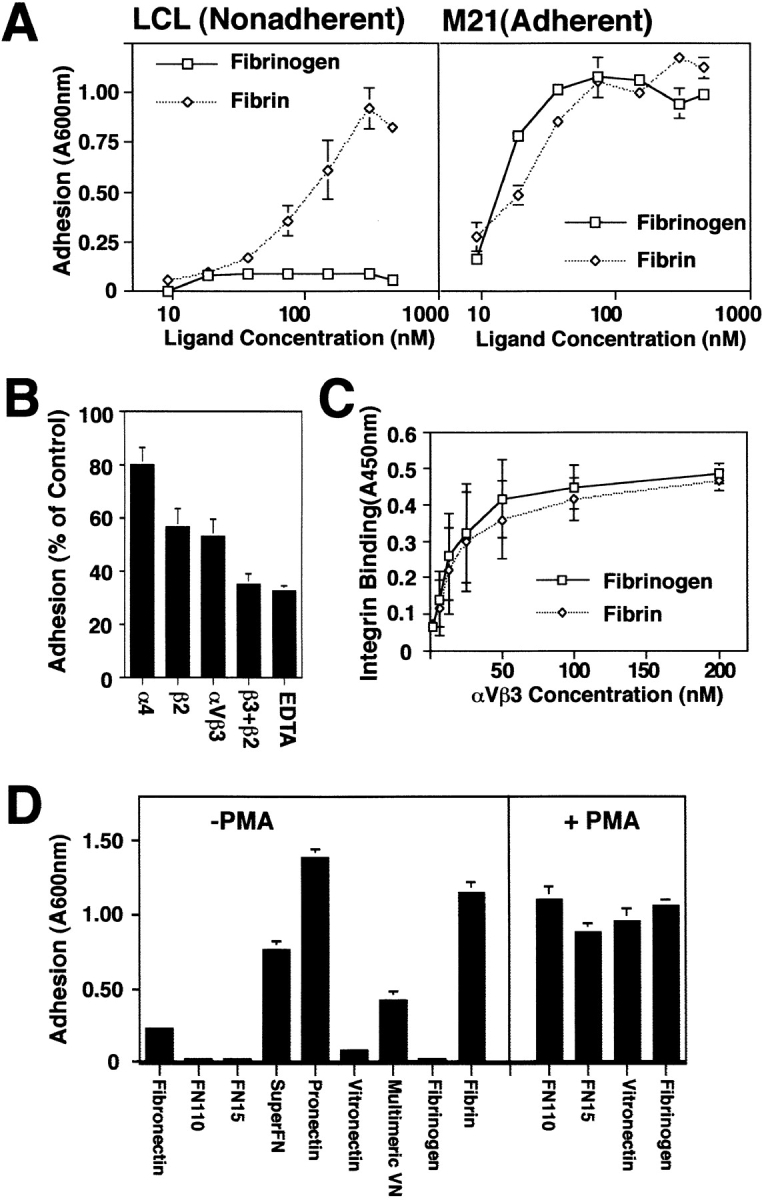
Preferential attachment of unactivated LCL to multimeric ECM components. (A) Nonadherent LCL or adherent M21 cells were allowed to attach to wells coated overnight with various concentrations of either fibrinogen or fibrin. Cells remaining attached after washing were stained with crystal violet and quantitated by reconstituting cell-bound dye with methanol and reading absorbance at 600 nm. The mean ± SE of triplicate determinations from one of three similar experiments is shown. (B) Attachment to polymerized fibrin was assessed in the presence of monoclonal antibodies specific for integrin α4 (P4C2), β2 (TS1/ 18), or αvβ3 (LM609) (25 μg/ml) or EDTA (10 mM) added to cell suspensions immediately before adhesion assay. (C) Relative affinity of the ligands for receptor was determined by binding of soluble, biotinylated integrin to fibrin or fibrinogen-coated wells that was detected by the addition of horseradish peroxidase–conjugated secondary anti-biotin monoclonal antibody as described in Materials and Methods. (D) Multivalent ECM ligands, including fibrin, superfibronectin (SuperFN), pronectin, and multimeric vitronectin (Multimeric VN) or unpolymerized/proteolyzed forms of these molecules forms including fibronectin fragments of 110 (FN110) and 15 kD (FN15), fibrinogen, and monomeric vitronectin (Vitronectin) were coated on adhesion assay wells overnight (0.5–5 μg/ml) and assessed for their capacity to support LCL adhesion (left). To determine if activation could rescue LCL adhesion to unpolymerized substrates, LCL were treated with PMA (20 ng/ml) immediately before adhesion assay on plates coated as described above (right). Representative results (mean ± SE of triplicate determinations) are shown; each substrate was tested three or more times.
