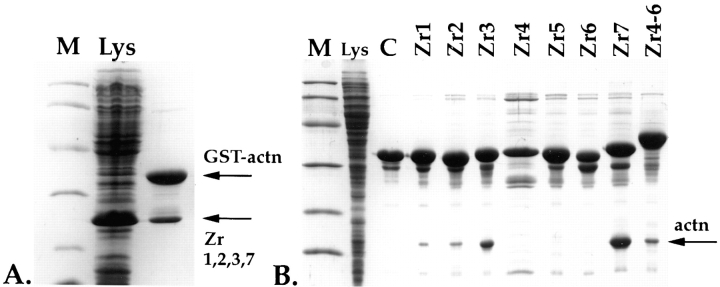
Figure 2.
In vitro interaction of Z-repeats with α-actinin. (A) Binding of four Z-repeat fragments, Zr1, 2, 3, and 7 to an α-actinin fusion protein. A Coomassie blue–stained 15% Laemmli SDS gel of a whole-cell lysate of BL21 cells expressing the Z-repeat fragment Zr1, 2, 3, and 7, which corresponds to the splice variant of psoas muscle (lane Lys). These cells were mixed with cells expressing a GST-His double-tagged α-actinin (GST-actn) fusion peptide and lysed together, and lysates were passed over Ni-NTA columns. Interaction of α-actinin with the Z-repeats is indicated by also retaining the nontagged Z-repeats on an Ni-NTA column (arrow Zr1,2,3,7). (B) As in A, but here the Z-repeats were expressed as GST-His6 double-tagged fusions, and the expressed α-actinin COOH-terminal peptide was tagless. Interaction of the 15-kD from the α-actinin COOH terminus with the Z-repeats is indicated by retaining the α-actinin 15 kD band on the column (arrow actn). Here, Lys indicates a whole-cell lysate of BL21 cells overexpressing the α-actinin COOH terminus; control (C), Zr1-His6-GST fusion peptide alone loaded on the column, demonstrating that a 15-kD protein from E. coli does not bind nonspecifically on the column. Lanes Zr1–Zr7, interaction of each of the seven Z-repeats alone with the COOH terminus of α-actinin; lane Zr4-6, interaction of the three fused Z-repeats with the COOH terminus of α-actinin. The Z-repeats Zr1, 2, 3, and 7 alone are sufficient for binding to the α-actinin COOH terminus under the conditions used. The Zr4, 5, and 6 alone do not interact with the α-actinin COOH terminus, but as a fusion peptide, they also bind to the COOH terminus of α-actinin. Lane M, molecular mass markers corresponding to 97, 60, 40, 30, 20, and 15 kD.