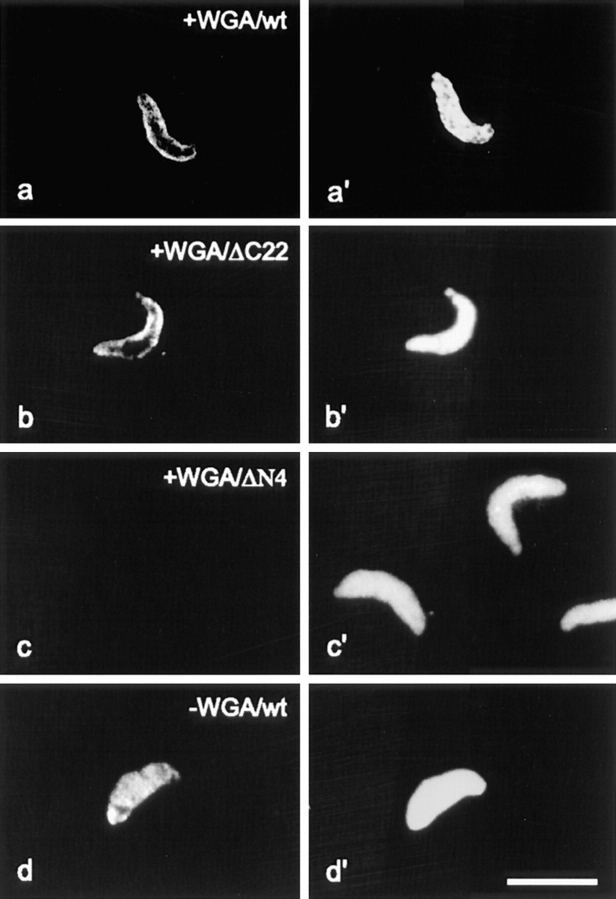
Figure 6.
The NH2-terminal, first element of HMG-NLS, is essential for docking of HMGs to the nuclear periphery. Wild-type (wt) recombinant HMG-17 or truncated versions of HMG-17 were added to Xenopus extract preincubated with WGA (see Materials and Methods). The localization of the added proteins was visualized by immunofluorescence (a–d). In a′–d′ corresponding DNA staining is shown. In the presence of WGA, wtHMG-17 and a truncation mutant containing both elements of the NLS dock to the pores as visualized by the ring-like staining around the nucleus (a and b). In contrast, a truncation mutant lacking the first four amino acids (i.e., the fist NLS element) fails to bind to the nuclear pores (c). In the absence of WGA, HMG17 enters the nucleus (d). Bar represents 10 μm.