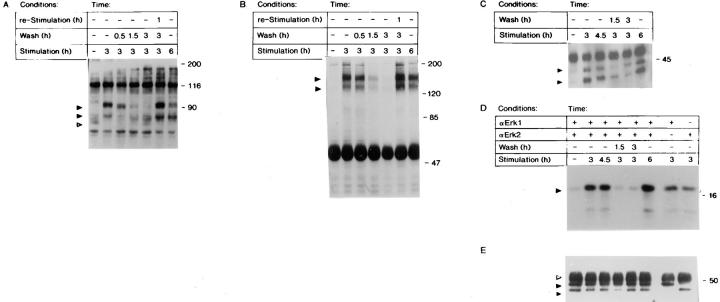
Figure 4.
(A) Immunoblot analysis of tyrosine phosphorylation in Swiss 3T3 cells after removal of FGF-1. Quiescent Swiss 3T3 cells were exposed to FGF-1 for 0, 3, or 6 h; stimulated with FGF-1 for 3 h, and washed with heparin and harvested 0.5, 1.5, or 3 h after the heparin wash; or were stimulated with FGF-1 for 3 h, washed with heparin, incubated in DMI without FGF-1 for 3 h, and then restimulated with FGF-1 for 1 h. The cells were harvested, and cytosolic lysates were prepared as described in Materials and Methods. Lysates were analyzed by 7.5% (wt/vol) SDS-PAGE, transferred to nitrocellulose membranes, and probed with an antiphosphotyrosine antibody as described (19). The positions of the p90 and p80 bands are indicated by closed arrows, and the p70 band is indicated by an open arrow. (B) Tyrosine phosphorylation of FGFR-1 in response to transient exposure to FGF-1. Quiescent Swiss 3T3 cells were stimulated, washed, and restimulated with FGF-1 in a manner identical to that described above. Cell lysates were immunoprecipitated with a rabbit anti-FGFR-1 polyclonal antibody and subjected to immunoblot analysis (7.5% [wt/vol] SDS-PAGE) with an antiphosphotyrosine antibody as described in Materials and Methods. The position of p130/p145 FGFR-1 polypeptides are indicated by closed arrows. (C) Tyrosine phosphorylation of p44mapk and p42mapk in response to transient exposure to FGF-1. Quiescent Swiss 3T3 cells were stimulated, washed, and restimulated with FGF-1 in a manner identical to that described in Fig. 4 A. Cell lysates were immunoprecipitated with a rabbit anti-ERK-1 polyclonal and rabbit anti-ERK-2 polyclonal antibodies, and were subjected to immunoblot analysis (9% [wt/ vol] SDS-PAGE) with an antiphosphotyrosine antibody as described in Materials and Methods. The positions of p44mapk (ERK-1) and p42mapk (ERK-2) are indicated by closed arrows. (D) In vitro MAP kinase activity in response to transient exposure to FGF-1. Quiescent Swiss 3T3 cells were either stimulated with FGF-1 for 0, 3, 4.5, or 6 h, or were stimulated with FGF-1 for 3 h, washed with heparin, and harvested 1.5 or 3 h after the heparin wash. Cell lysates were immunoprecipitated with anti-Erk-1 and/or anti-Erk-2, and an in vitro kinase assay was performed using MBP as the substrate. The kinase reactions were analyzed by 12.5% (wt/vol) SDS-PAGE, transferred to nitrocellulose membranes, and phosphorylated MBP was visualized by autoradiography. The position of MBP is indicated by a closed arrow. Because the p44mapk band does not resolve away from the antibody band in the immunoblot, we used a different experiment in which a similar trend was observed to quantitate these results: quiescence, 1.0; 3-h stimulation, 14.9; 4.5-h stimulation, 16.4; 3-h stimulation followed by a 1.5-h withdrawal, 3.7; 3-h stimulation followed by a 3-h withdrawal, 2.0; and 6-h stimulation, 24.5. (E) The level of p44mapk (ERK-1) and p42mapk (ERK-2) protein was visualized by immunoblot analysis with p44mapk (ERK-1) polyclonal antibodies. The position of p44mapk and p42mapk is indicated by a closed arrow, and the position of IgG is indicated with an open arrow.