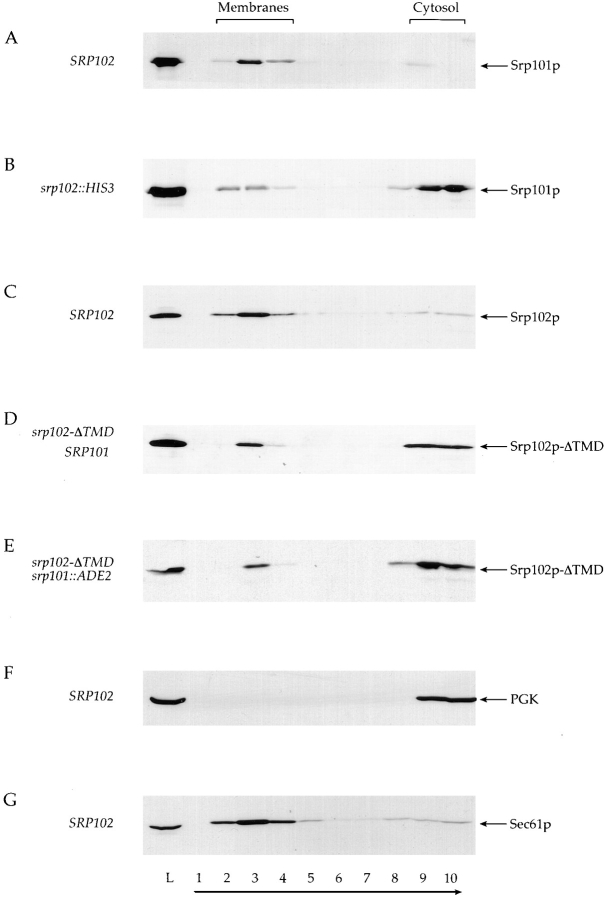
Figure 6.
Fractionation behavior of Srp101p and Srp102 during perturbation of the SR. Whole-cell lysate from cells was layered under a 2.4-ml discontinuous sucrose gradient, and was centrifuged to equilibrium. Equivalent amounts of the load fraction (lane L) and fractions from the gradient (lanes 1–10) were analyzed by Western blot using anti-Srp101p, anti-Srp102p, anti-PGK, or anti-Sec61p antisera. Fractionation behavior of Srp101p was compared between strains containing either wild-type Srp102p ([A] SRP102, WBY618 bearing pSO452) or the srp102:: HIS3 deletion ([B], WBY618). As controls, fractionation of the cytosolic enzyme phosphoglucokinase ([F] PGK) or the integral ER membrane protein Sec61p (G) was also monitored in a strain wild-type for SRP102 (WBY618 bearing pSO452) to identify those fractions containing cytosol or membranes. Srp102p fractionation was also monitored in wild-type cells ([C] SRP102, WBY618 bearing pSO452) as was fractionation of Srp102p-ΔTMD in cells either containing or lacking Srp101p ([D] WBY618 bearing pWB209; [E] WBY823). Direction of sedimentation is indicated by an arrow, and the fractions containing cytosol (lanes 9 and 10) or membranes (lanes 2–4) are also indicated above.