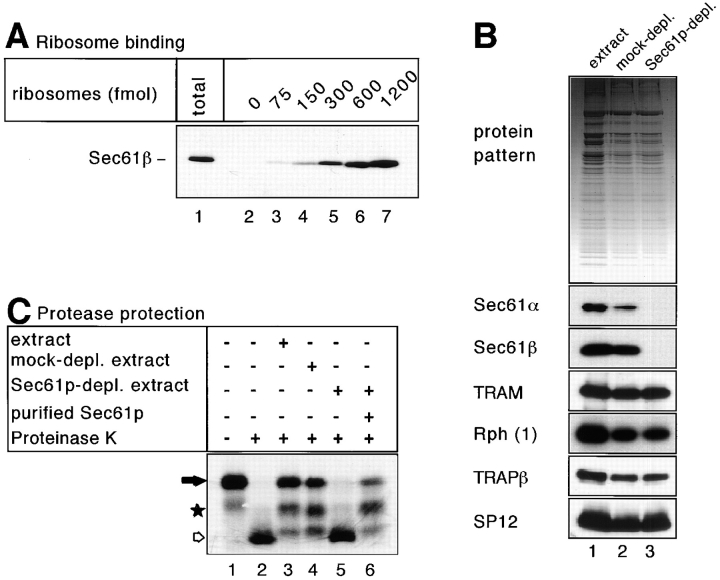
Figure 2.
Sec61p is essential for protease protection of nascent preprolactin chains in the soluble system. (A) Nontranslating ribosomes in the indicated amounts were incubated with a detergent extract of microsomes (corresponding to ∼5–7 pmol Sec61p). After sedimentation of the ribosomes through a sucrose cushion, the amount of cosedimenting Sec61p complex was determined by immunoblotting with antibodies against the β-subunit of the Sec61p complex (Sec61β). Lane 1 shows the total amount of Sec61p complex added to the ribosomes. (B) The upper part shows the protein pattern of an untreated detergent extract, of a mock-immunodepleted extract after incubation with protein A/G–Sepharose (mock-depl.), and of an extract immunodepleted with antibodies to Sec61β that had been coupled to protein A/G– Sepharose (Sec61p-depl.). The lower part shows immunoblots of the same samples using antibodies against Sec61α, Sec61β, TRAM, ribophorin 1 (Rph 1), the β subunit of the translocon- associated protein complex (TRAPβ), and the 12-kD subunit of signal peptidase (SP12). (C) RNCs containing 86 amino acids of preprolactin were incubated with the extracts shown in (B) and treated with proteinase K. In lane 6, purified Sec61p complex was added in amounts corresponding to those in the original sample. The positions of the nascent chains (filled arrow) and of their proteolytic fragments (star and hollow arrow) are indicated.