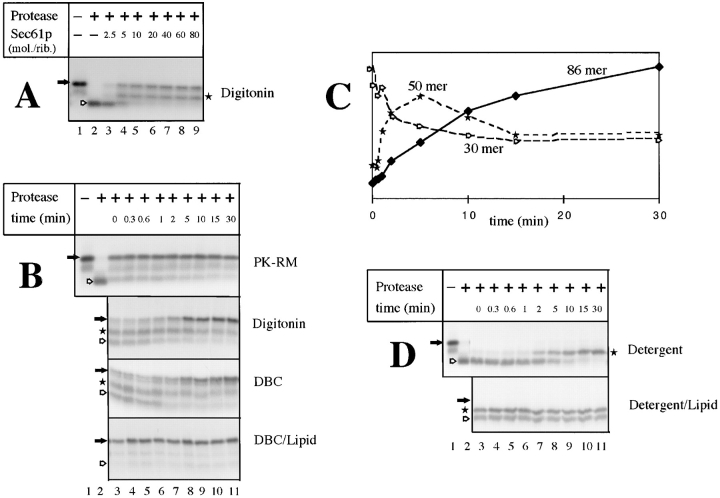
Figure 3.
Insertion of nascent preprolactin chains into the purified, soluble Sec61p channel. (A) Isolated RNCs (∼0.2 pmol ribosomes) carrying preprolactin chains of 86 amino acids were incubated for 15 min at 26°C with different amounts of purified Sec61p complex in 1% digitonin. The samples were then treated with proteinase K and analyzed by SDS-PAGE. The positions of the nascent chains (filled arrow) and of the proteolytic fragments of 30 (hollow arrow) and of 50 (star) amino acids are indicated. (B) Isolated RNCs were incubated at 26°C for different time periods with either ribosome-stripped canine pancreatic microsomes (PK-RM), or with purified Sec61p complex in either 1% digitonin, 0.3% deoxyBigCHAP (DBC), or a mixture of 0.6% deoxyBigCHAP and 2 mg/ ml phospholipids (DBC/Lipid; approximate molar ratio of 80 mol of Sec61p complex per mole of ribosomes). The samples were treated with proteinase K and analyzed by SDS-PAGE. Filled arrows, hollow arrows, and stars indicate the positions of the nondegraded nascent chains, and of the fragments of 30 and 50 amino acids, respectively. (C) An experiment in digitonin, similar to that in the second panel of B, was performed with a Sec61p/ribosome molar ratio of 10 and quantitated to demonstrate that the fragment of 50 amino acids corresponds to a kinetic intermediate. (D) RNCs containing 83 amino acids of a signal sequence mutant of preprolactin (pPLΔ13-15) were incubated for different time periods at 26°C with purified Sec61p in either digitonin or a deoxyBigCHAP–lipid mixture (DBC/Lipid; molar ratio of Sec61p complex to ribosomes of 10), and treated with proteinase K. The positions of the nascent chains (filled arrow) and of their proteolytic fragments (hollow arrow and star) are indicated.