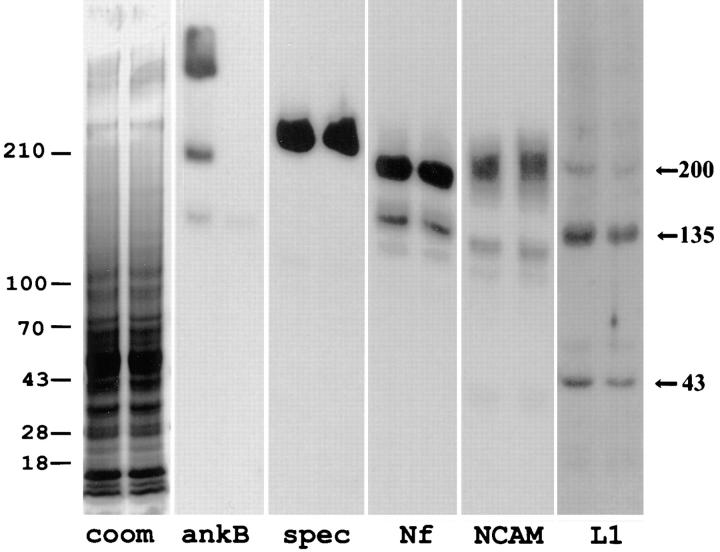
Figure 7.
Immunoblots of neurofascin, NCAM, βG spectrin, and L1 in brain tissue from ankyrinB (+/+) and (−/−) mice. Polypeptides in total brain homogenates of an ankyrinB mouse (−/−) (left lanes) and a (+/+) littermate (right lanes) mouse were separated on a 3.5–17.5% SDS–polyacrylamide gel and either stained with Coomassie blue or transferred to a nitrocellulose membrane. Duplicate membranes were blotted with polyclonal antibodies to ankyrinB (ankB), spectrin (spec), neurofascin (Nf), NCAM, and L1, and bound antibodies were detected with 125I-labeled protein A. L1 polypeptides of 200, 135, and 43 kD are marked on the left. The extent of labeling was quantitated by a phosphoimager. Immunoblots were scanned to a phosphoimager and analyzed using ImageQuant software developed by Molecular Dynamics (Sunnyvale, CA). Compared with the wild-type littermate, L1 polypeptides of 200, 135, and 43 kD were reduced by 38, 29, and 27%, respectively, in ankyrinB (−/−) brain samples. The relative amounts of spectrin, neurofascin, and NCAM in the ankyrinB (−/−) samples were 0.95, 1.01, and 1.06 that of wild-type samples, respectively.