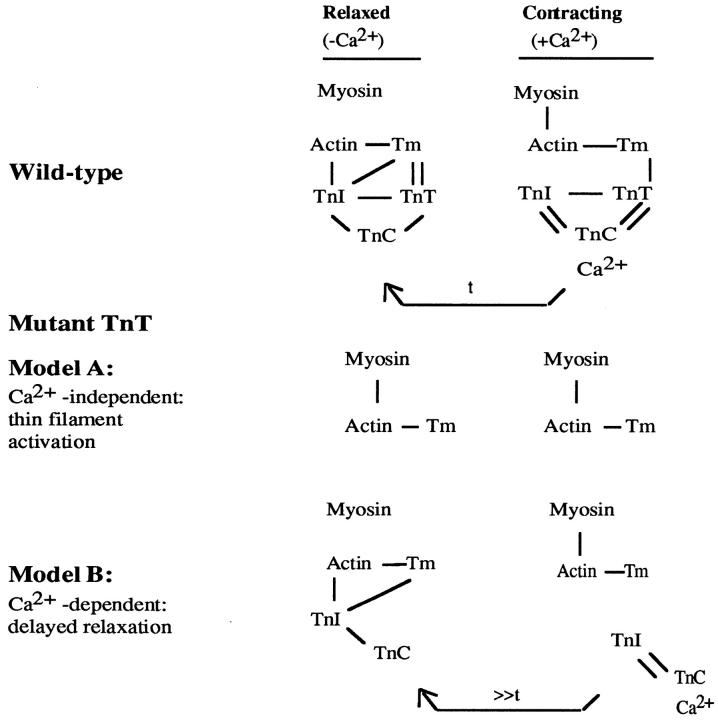
Figure 3.
Model for associations of muscle proteins. This figure presents a simplified view of how the thin filament proteins function based on biochemical studies (adapted from 36). TnI is proposed to be capable of interacting with TnC independent of TnT. The relationship of these interactions in vivo is uncertain although there are two simple hypotheses. The first hypothesis, consistent with biochemical studies of reconstituted thin filaments, would be that TnI in vivo cannot associate with the thin filament in the absence of TnT (Model A). The second hypothesis, consistent with biochemical studies of troponin subunit interactions, would be that TnI and TnC, in vivo, can interact with actin and inhibit contraction under conditions of low Ca2+ in the absence of the TnT tethering function, albeit less efficiently, i.e., it would take longer (>>t) to reestablish the inhibited state (Model B, delayed relaxation). If the first hypothesis were true, one would predict that the Mup phenotype should be Ca2+-independent, since TnI and TnC would never associate with the thin filament, and hence the filament would be constantly activated. Our data are consistent with Model B.