Figure 1.
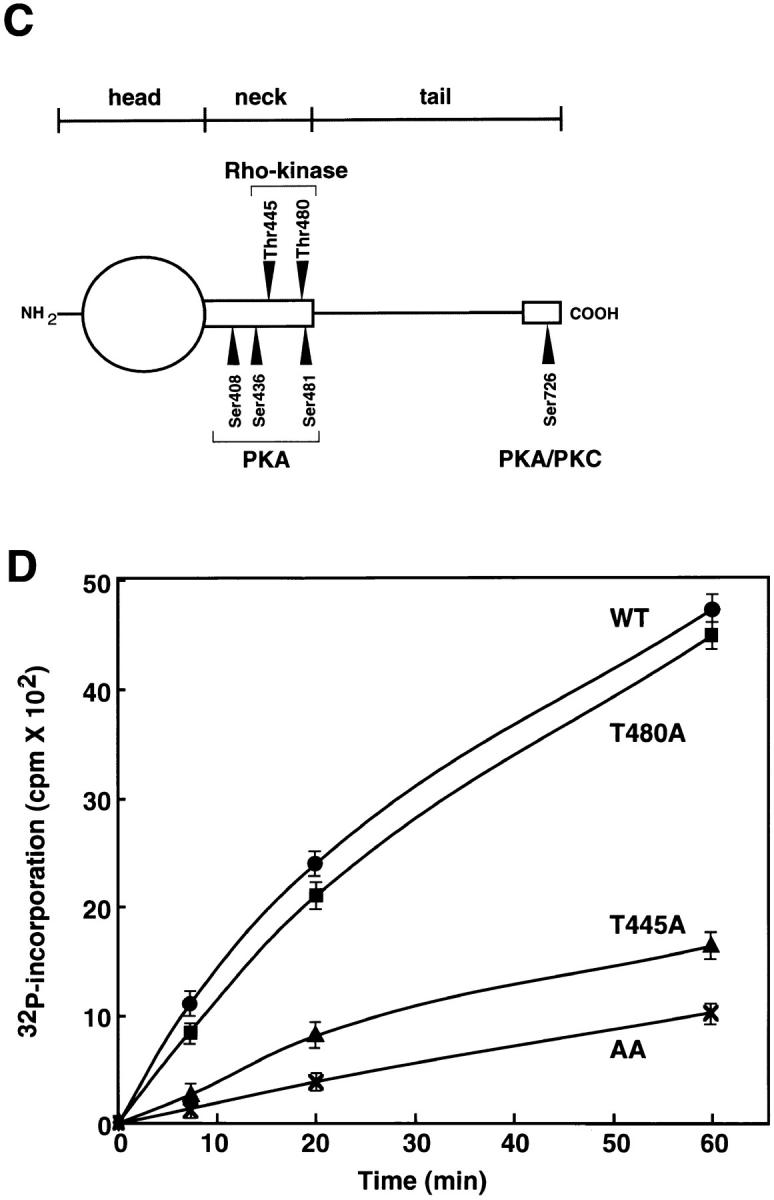
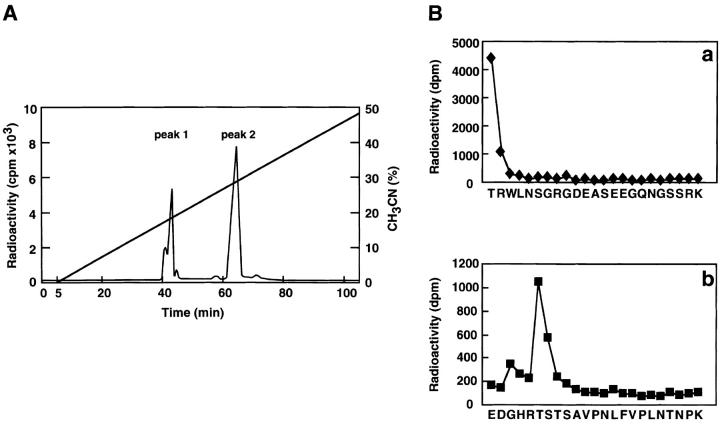
Determination of sites of phosphorylation of α-adducin by Rho-kinase. (A) Reverse-phase HPLC for AP-1 fragments of α-adducin phosphorylated by Rho-kinase was carried out under the conditions described in Materials and Methods. CH3CN, acetonitrile. (B) Amino acid sequences of phosphopeptides derived from α-adducin phosphorylated by Rho-kinase. The radioactive peaks 1 (panel a) and 2 (panel b) were separated. Phosphoamino acid sequencing of each peak was carried out with a peptide sequencer, and the fractions obtained from each Edoman degradation cycle were measured for 32P in a liquid scintillation counter. (C) Structure and phosphorylation sites of α-adducin. A model for α-adducin based on Matsuoka et al. (1996) is presented. The phosphorylation sites of α-adducin for PKA, PKC, and Rho-kinase are presented. (D) Preferential phosphorylation of α-adducin at Thr445 by Rho-kinase in vitro. GST-α-adducin (WT, circles), GST-α-adducin-AA (AA, X), GST-α-adducinT445A (T445A, triangles), and GST-α-adducinT480A (T480A, squares) were phosphorylated by GST-CAT for various periods. Data are means ± SEM of triplicate determinations.