Figure 5.
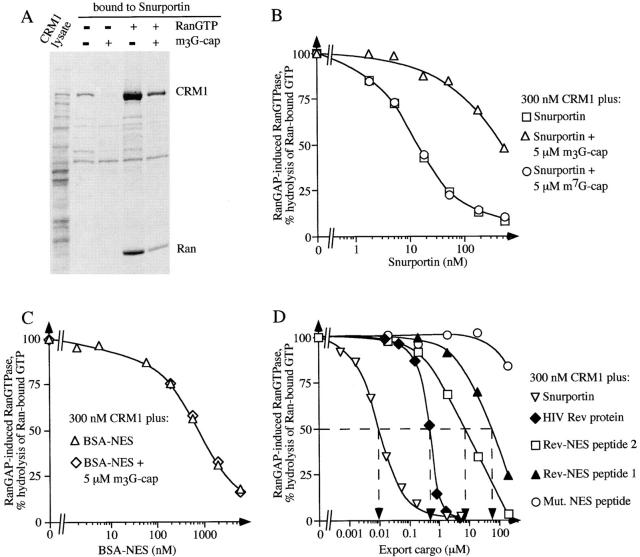
Binding of m3G-cap and of CRM1 to snurportin 1 are mutually exclusive. (A) Immobilized snurportin 1 was used to bind recombinant CRM1 out of total E. coli lysate either in the presence (lanes 3 and 5) or absence (lanes 2 and 4) of a 10-fold molar excess of a m3G-cap oligonucleotide (5 μM). Where indicated, 11 μM RanQ69L was also added (lanes 4 and 5). In the absence of Ran-GTP, m3G-cap prevented binding of CRM1 to snurportin, while in the presence of Ran-GTP binding of CRM1 to snurportin 1 is reduced but not abolished by m3G-cap. (B) Formation of the trimeric snurportin/CRM1/ RanGTP complex was measured as in Fig. 2 A, with the modification that the concentration of CRM1 was kept constant at 300 nM and the concentration of snurportin 1 was varied. Where indicated, snurportin 1 had been preincubated with either 5 μM m3G-capped oligonucleotide or m7GpppG dinucleotide. Note that the m3G-cap RNA oligonucleotide specifically inhibited trimeric complex formation, while the m7G-cap analogue had no effect. (C) Measurements were performed exactly as in B except that a Rev-NES-BSA conjugate was used instead of snurportin. Note that the m3G-cap RNA oligonucleotide had no effect on the Rev-NES/CRM1/RanGTP interaction. (D) Measurements were performed exactly as in B, varying the concentrations of the following export substrates: snurportin 1; HIV Rev protein; Rev-NES peptide 1, which is a synthetic peptide (cys-LPPLERLTL) corresponding to the minimum Rev activation domain, Rev-NES peptide 2, which is a slightly larger peptide (cys-PVPLQLPPLERLTLD) that also includes NH2- and COOH-terminally flanking residues, Mut. NES peptide (cys-LPPDLRLTL), which corresponds to a loss-of-function mutant of the activation domain, was used as a negative control.