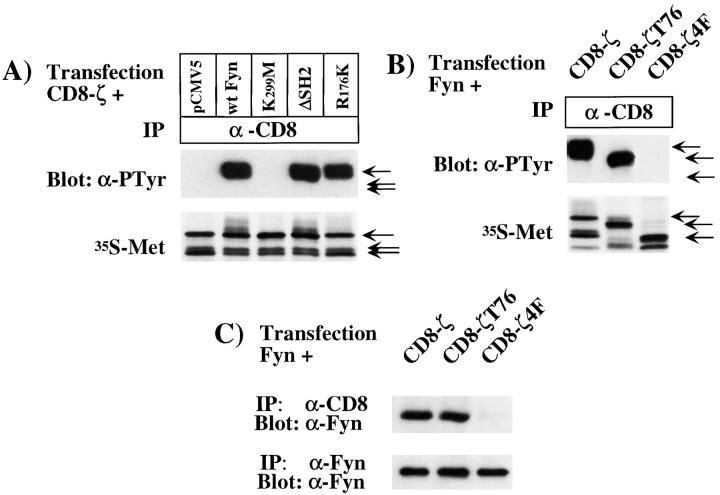
Figure 8.
Analysis of the molecular interactions between Fyn and CD8-ζ. (A) COS-1 cells were transfected with cDNAs encoding CD8-ζ and either pCMV5, wild-type Fyn, K299M-Fyn, ΔSH2-Fyn, or R176K-Fyn. Transfected cells were lysed in RIPA buffer and were subjected to immunoprecipitation with anti– CD8 antibody, followed by Western blotting analysis using antiphosphotyrosine (α-PTyr) monoclonal antibody (top). As controls for expression levels, transfected cells were radiolabeled with Tran35S-label (bottom), lysed in RIPA buffer, and subjected to immunoprecipitation with anti–CD8 antibody, followed by SDS-PAGE and autoradiography. CD8-ζ expressed in COS-1 cells is present in three forms that migrate at 36, 38, and 41 kD, respectively, as indicated by the arrows. The 41-kD form represents the main tyrosine phosphorylated band. Overexposure of the blot in the top row revealed a low level of phosphorylation of CD8-ζ in the pCMV5 and K299M lanes, presumably reflecting the activity of endogenous Fyn or other kinases. (B) COS-1 cells were transfected with cDNAs encoding wild-type Fyn and either CD8-ζ, CD8-ζT76, or CD8-ζ4F. Antiphosphotyrosine Western blotting and radiolabeling were performed as described in A. Truncated CD8-ζT76 was present in one main form that migrated at ∼40 kD while the main radiolabeled form of CD8-ζ4F migrated at ∼35 kD, as indicated by the arrows. (C) COS-1 cells were transfected as described in B and coimmunoprecipitation analysis of Fyn with CD8-ζ constructs was performed as described in Fig. 7 C, legend.