Figure 8.
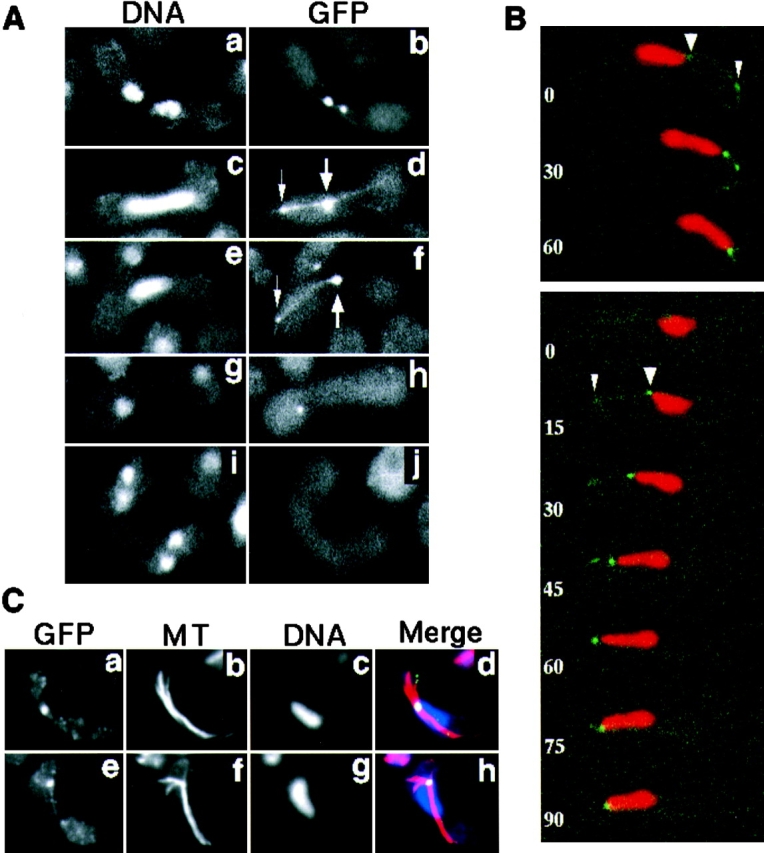
Localization of GFP-tagged dynein during karyogamy and meiosis. (A) Localization of dynein-GFP in living cells. Homothallic cells bearing the dynein-GFP fusion gene (strain CRL1526) were induced into meiosis and observed for localization of dynein-GFP. Chromosomal DNA was stained with Hoechst 33342. Cells undergoing karyogamy (a and b), the oscillatory nuclear movement (c–f), first meiotic division (g and h), and second meiotic division (i and j) are shown. a, c, e, g, and i, DNA staining; b, d, f, h, and j, GFP signal. The large arrows indicate the GFP dot at the leading edge of the moving nucleus where the SPB is expected. The small arrows indicate a GFP dot at a point where a GFP line contacts the cell cortex. (B) Time-lapse series of meiotic cells expressing dynein-GFP. Two zygotic cells undergoing the oscillatory nuclear movement are shown. Red and green indicate DNA staining and GFP signal, respectively. Numbers indicate time in seconds. Large and small arrowheads indicate the GFP dots at the leading edge of the moving nucleus and at the point where a GFP line contacts the cell cortex, respectively. (C) Immunofluorescence staining of dynein-GFP and microtubules in fixed cells. Cells induced to meiosis, as in A, were fixed and stained using antibodies against GFP (a and e) and α-tubulin (b and f). They were also stained with DAPI (c and g). Merged images are shown in d and h. Yellow/green, GFP; red, tubulin; and blue, DNA staining.
