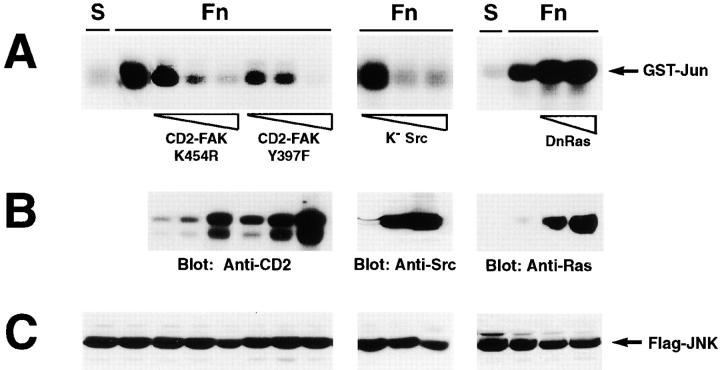
Figure 3.
Role of the FAK/Src complex in integrin-mediated activation of JNK. 293 cells were transiently transfected with 3 μg of vector encoding FLAG-tagged JNK alone or in combination with 3, 9, and 27 μg of plasmids encoding CD2-FAK K454R (catalytically inactive), CD2-FAK Y397F (unable to bind to the SH2 domain of Src family kinases), and K− Src (kinase dead), or with 9 and 27 μg of vector encoding DnRas (N17, dominant-negative). The cells were serum starved for 24 h, detached, and lysed immediately (S) or plated on fibronectin for 20 min (Fn). FLAG-tagged JNK was immunoprecipitated from extracts containing equal amounts of total proteins and subjected to an in vitro kinase assay with GST-Jun as a substrate (A). Total lysates containing the same amount of proteins were subjected to immunoblotting with antibodies to CD2, Src, and Ras to verify that the expression of the various dominant-negative proteins was proportional to the amount of DNA transfected (B). Immunoblotting with anti-FLAG antibodies was used to verify equal expression of FLAG-JNK in all samples (C).