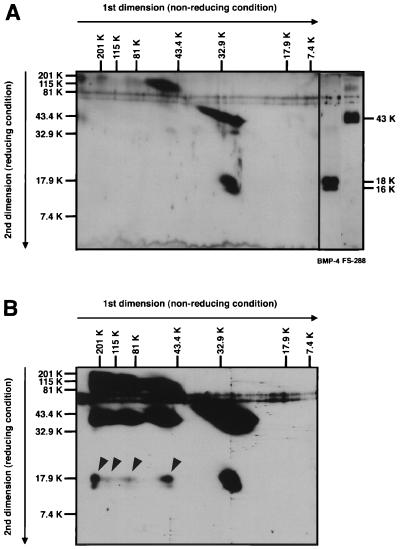
Figure 4.
Diagonal SDS/PAGE analysis of FS-288-BMP-4 complex. (A) For comparison of molecular mass, a mixture of FS-288 and BMP-4 was analyzed by 2D electrophoresis. Although FS-288 (32 kDa) tends to shift to higher molecular mass region (>60 kDa) on first dimension electrophoresis probably by self aggregation, a major band of BMP-4 shows the estimated molecular mass of dimeric form (30 kDa). FS-288 was detected as a 43-kDa protein, and BMP-4 was detected as 16- and 18-kDa proteins, which were generated because of difference of glycosylation, under reducing condition. (B) Cross-linked FS-288-BMP-4 complex was analyzed. Proteins were separated by 2D electrophoresis and subjected to Western blotting. At least, four-shifted bands (arrowheads) of BMP-4, which were not seen in A, were detected. Relative molecular mass is indicated horizontally for nonreducing, vertically for reducing condition.