Abstract
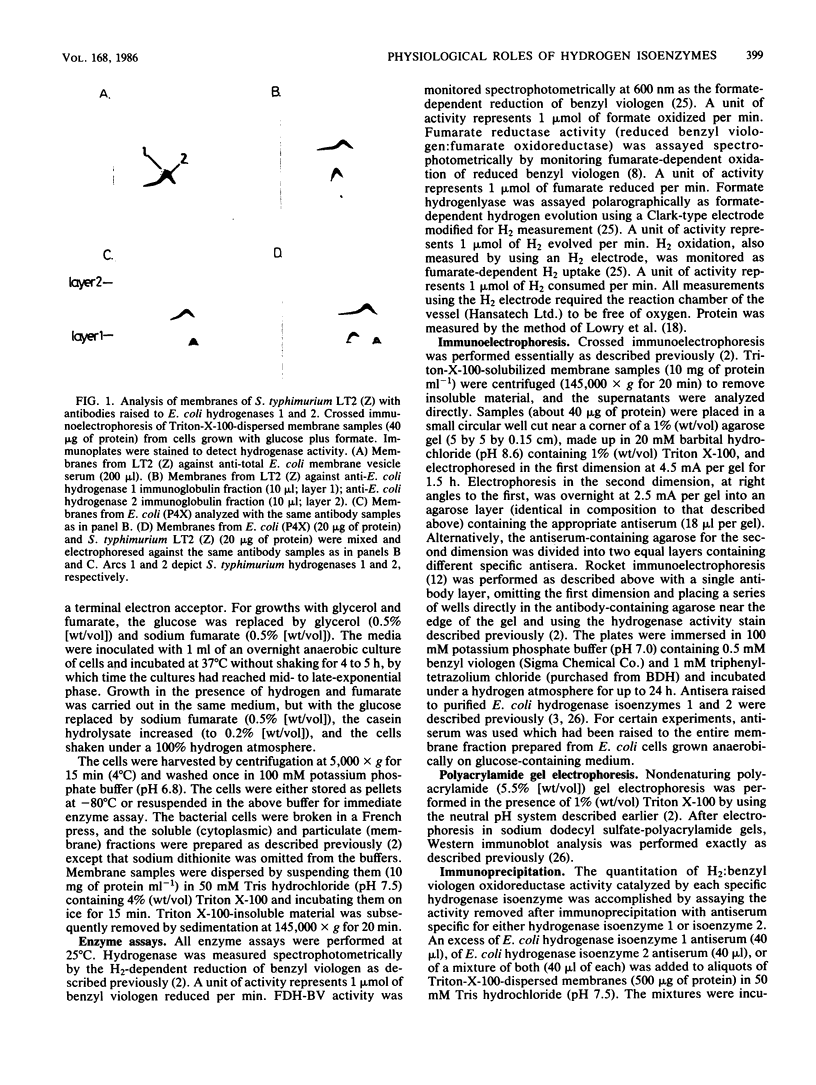
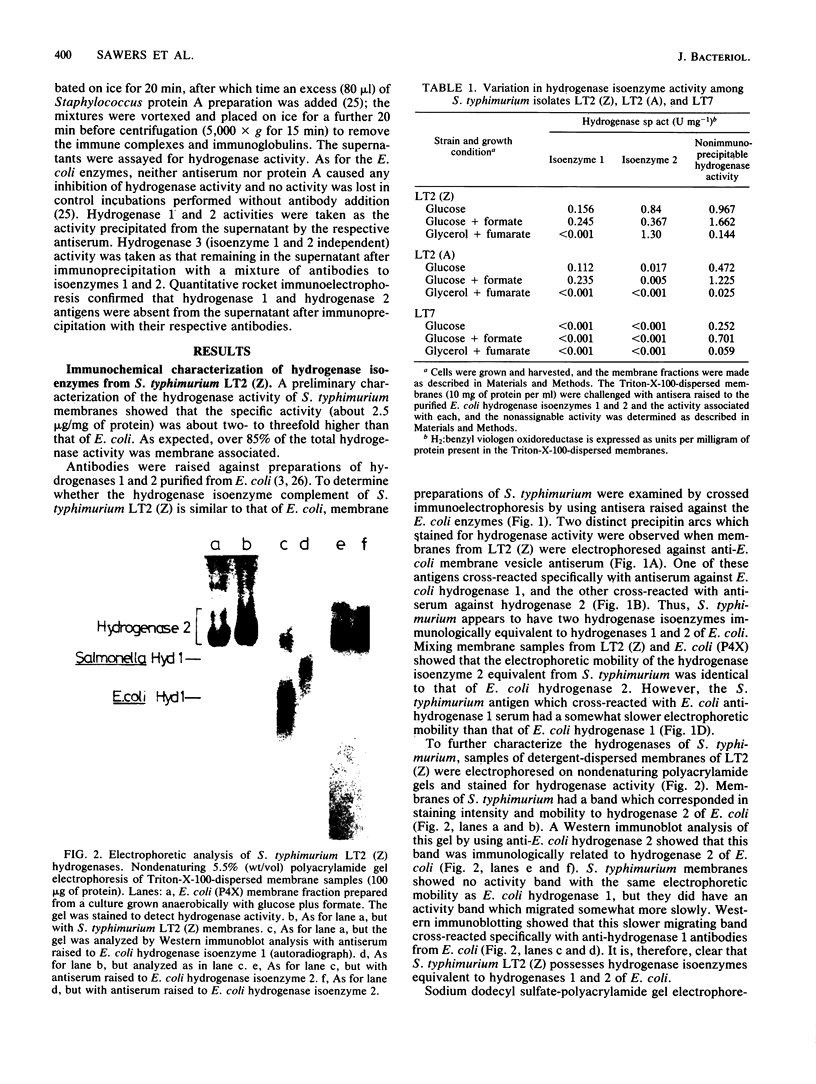
We found that Salmonella typhimurium strain LT2 (Z) possessed two immunologically distinct, membrane-bound hydrogenase isoenzymes, which were similar in electrophoretic mobilities and apoprotein contents to hydrogenase isoenzymes 1 and 2 of Escherichia coli. The S. typhimurium enzymes cross-reacted with antibodies raised to the respective hydrogenase isoenzymes of E. coli. As for E. coli, an additional membrane-bound hydrogenase activity (termed hydrogenase 3), which did not cross-react with antibodies raised against either hydrogenase 1 or 2, was also present in detergent-dispersed membrane preparations. The physiological role of each of the three isoenzymes in E. coli has remained unclear owing to the lack of mutants specifically defective for individual isoenzymes. However, analysis of two additional wild-type isolates of S. typhimurium revealed specific defects in their hydrogenase isoenzyme contents. S. typhimurium LT2 (A) lacked isoenzyme 2 but possessed normal levels of hydrogenases 1 and 3. S. typhimurium LT7 lacked both isoenzymes 1 and 2 but retained normal hydrogenase 3 activity. Characterization of hydrogen metabolism by these hydrogenase-defective isolates allowed us to identify the physiological role of each of the three isoenzymes. Hydrogenase 3 activity correlated closely with formate hydrogenlyase-dependent hydrogen evolution, whereas isoenzyme 2 catalyzed hydrogen uptake (oxidation) during anaerobic, respiration-dependent growth. Isoenzyme 1 also functioned as an uptake hydrogenase but only during fermentative growth. We postulate that this enzyme functions in a hydrogen-recycling reaction which operates during fermentative growth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W., Mortenson L. E., Chen J. S. Hydrogenase. Biochim Biophys Acta. 1980 Dec;594(2-3):105–176. doi: 10.1016/0304-4173(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Ballantine S. P., Boxer D. H. Isolation and characterisation of a soluble active fragment of hydrogenase isoenzyme 2 from the membranes of anaerobically grown Escherichia coli. Eur J Biochem. 1986 Apr 15;156(2):277–284. doi: 10.1111/j.1432-1033.1986.tb09578.x. [DOI] [PubMed] [Google Scholar]
- Ballantine S. P., Boxer D. H. Nickel-containing hydrogenase isoenzymes from anaerobically grown Escherichia coli K-12. J Bacteriol. 1985 Aug;163(2):454–459. doi: 10.1128/jb.163.2.454-459.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Cole J. A. Microbial gas metabolism. Adv Microb Physiol. 1976;14(11):1–92. doi: 10.1016/s0065-2911(08)60226-x. [DOI] [PubMed] [Google Scholar]
- Dickie P., Weiner J. H. Purification and characterization of membrane-bound fumarate reductase from anaerobically grown Escherichia coli. Can J Biochem. 1979 Jun;57(6):813–821. doi: 10.1139/o79-101. [DOI] [PubMed] [Google Scholar]
- Glaser J. H., DeMoss J. A. Comparison of nitrate reductase mutants of Escherichia coli selected by alternative procedures. Mol Gen Genet. 1972;116(1):1–10. doi: 10.1007/BF00334254. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H., Haddock B. A., Mandrand-Berthelot A. M., Jones R. W. Immunochemical analysis of the membrane-bound hydrogenase of Escherichia coli. FEBS Lett. 1980 May 5;113(2):167–172. doi: 10.1016/0014-5793(80)80584-9. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. The organization of formate dehydrogenase in the cytoplasmic membrane of Escherichia coli. Biochem J. 1981 Jun 1;195(3):627–637. doi: 10.1042/bj1950627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Higgins C. F. Two genetically distinct pathways for transcriptional regulation of anaerobic gene expression in Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):389–397. doi: 10.1128/jb.168.1.389-397.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Sawers R. G., Rugman P. A., Boxer D. H., Higgins C. F. Effects of anaerobic regulatory mutations and catabolite repression on regulation of hydrogen metabolism and hydrogenase isoenzyme composition in Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):405–411. doi: 10.1128/jb.168.1.405-411.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W. The role of the membrane-bound hydrogenase in the energy-conserving oxidation of molecular hydrogen by Escherichia coli. Biochem J. 1980 May 15;188(2):345–350. doi: 10.1042/bj1880345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambden P. R., Guest J. R. Mutants of Escherichia coli K12 unable to use fumarate as an anaerobic electron acceptor. J Gen Microbiol. 1976 Dec;97(2):145–160. doi: 10.1099/00221287-97-2-145. [DOI] [PubMed] [Google Scholar]
- Lee J. H., Patel P., Sankar P., Shanmugam K. T. Isolation and characterization of mutant strains of Escherichia coli altered in H2 metabolism. J Bacteriol. 1985 Apr;162(1):344–352. doi: 10.1128/jb.162.1.344-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy J., Kulla H., Gottschalk G. H2-dependent anaerobic growth of Escherichia coli on L-malate: succinate formation. J Bacteriol. 1976 Feb;125(2):423–428. doi: 10.1128/jb.125.2.423-428.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom J. M., Peck H. D., Jr Hydrogenase, electron-transfer proteins, and energy coupling in the sulfate-reducing bacteria Desulfovibrio. Annu Rev Microbiol. 1984;38:551–592. doi: 10.1146/annurev.mi.38.100184.003003. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. Formic dehydrogenase and the hydrogenlyase enzyme complex in coli-aerogenes bacteria. J Bacteriol. 1957 Jun;73(6):706–721. doi: 10.1128/jb.73.6.706-721.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal M. C., Casse F., Chippaux M., Lepelletier M. Genetic analysis of mutants of Escherichia coli K12 and Salmonella typhimurium LT2 deficient in hydrogenase activity. Mol Gen Genet. 1975 Nov 24;141(2):173–179. doi: 10.1007/BF00267682. [DOI] [PubMed] [Google Scholar]
- Sawers R. G., Ballantine S. P., Boxer D. H. Differential expression of hydrogenase isoenzymes in Escherichia coli K-12: evidence for a third isoenzyme. J Bacteriol. 1985 Dec;164(3):1324–1331. doi: 10.1128/jb.164.3.1324-1331.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers R. G., Boxer D. H. Purification and properties of membrane-bound hydrogenase isoenzyme 1 from anaerobically grown Escherichia coli K12. Eur J Biochem. 1986 Apr 15;156(2):265–275. doi: 10.1111/j.1432-1033.1986.tb09577.x. [DOI] [PubMed] [Google Scholar]
- Vignais P. M., Colbeau A., Willison J. C., Jouanneau Y. Hydrogenase, nitrogenase, and hydrogen metabolism in the photosynthetic bacteria. Adv Microb Physiol. 1985;26:155–234. doi: 10.1016/s0065-2911(08)60397-5. [DOI] [PubMed] [Google Scholar]
- Waugh R., Boxer D. H. Pleiotropic hydrogenase mutants of Escherichia coli K12: growth in the presence of nickel can restore hydrogenase activity. Biochimie. 1986 Jan;68(1):157–166. doi: 10.1016/s0300-9084(86)81080-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto I., Ishimoto M. Hydrogen-dependent growth of Escherichia coli in anaerobic respiration and the presence of hydrogenases with different functions. J Biochem. 1978 Sep;84(3):673–679. doi: 10.1093/oxfordjournals.jbchem.a132172. [DOI] [PubMed] [Google Scholar]