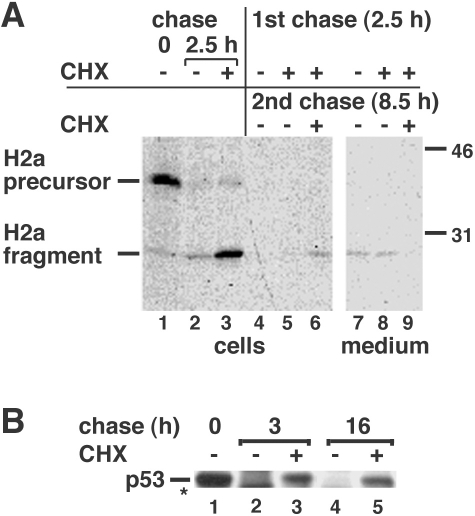
Fig 2.
Inhibition of protein synthesis blocks only transiently the degradation of H2a but extendedly that of p53. (A) NIH 3T3 cells stably expressing H2a (2–18 cell line) were metabolically labeled for 20 min with [35S] Cys and chased for 0 or 2.5 hours with complete medium in the absence or presence of 300 μM cycloheximide (CHX) as indicated. Some samples (lanes 4–9) then were rinsed and incubated for an additional 8.5 hours in the absence or presence of 300 μM CHX. At the end of the pulse or chase periods, the cells were lysed; the lysates (cells) or cell supernatants (medium) were immunoprecipitated with anti-H2a antibody, treated with N-glycosidase F, and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by fluorography. On the right are indicated molecular masses in kilodaltons. On the left are the migrations of H2a precursor and cleaved ectodomain fragment. (B) NIH 3T3 cells were metabolically labeled for 1 hour with [35S] Cys+Met mix and chased for the indicated times in the presence or absence of CHX. Cell lysates were immunoprecipitated with anti-p53 antibodies and analyzed by SDS-PAGE and fluorography. The asterisk indicates a cleavage product