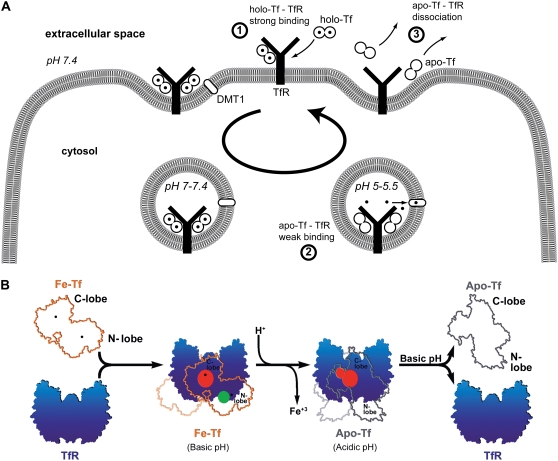
FIGURE 7.
Models of Tf-TfR interactions along the Tf cycle. (A) Variations of unbinding forces along the cycle. First, holo-Tf strongly binds TfR at the cell surface (unbinding force of 54 pN at 3.3 nN/s). Second, the complex is internalized and transported to acidic endosomes. Conformational changes trigger iron release (solid circles) from holo-Tf, which becomes apo-Tf and remains weakly bound to TfR (unbinding force of 43 pN at 3.5 nN/s). Third, the complex is brought back at the cell surface and apo-Tf dissociates from TfR. (B) The model adapted from Giannetti et al. for the binding of holo-Tf and apo-Tf to TfR (9). At the cell surface pH, holo-Tf (here named Fe-Tf, orange outline with iron ions marked as black dots) binds TfR (blue) through its C-lobe (red binding site) and through its N-lobe (green binding site). At endosomal pH, iron is released and conformational changes occur. Apo-Tf (gray outline) binds TfR through its C-lobe only (red binding site). Upon return to the cell surface, apo-Tf dissociates from TfR. The two binding sites of holo-Tf might explain the two barriers of the holo-Tf-TfR energy landscape (see Fig. 8), whereas the absence of binding between the apo-Tf N-lobe and TfR might explain the absence of an inner barrier in the apo-Tf-TfR energy landscape.