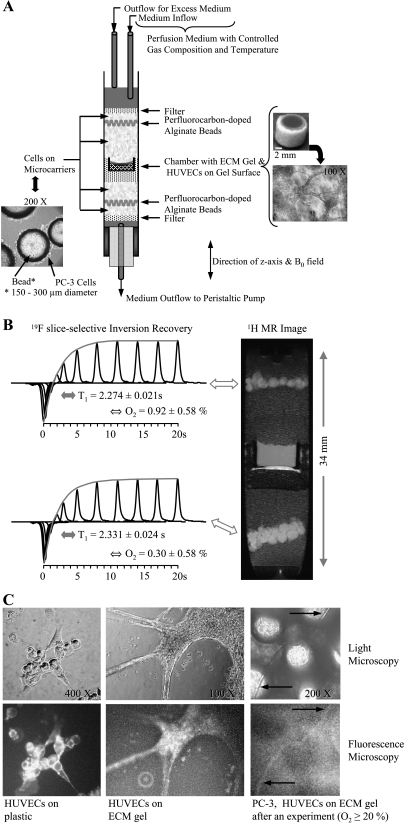
Figure 1.
(A) Photomicrograph of PC-3 cells grown adherently on microcarriers (left) and schematic display of the sample structure (center). On the right are photomicrographs of the invasion chamber filled with ECM gel (top) and an example of the distribution of HUVECs on the ECM gel at the start of an experiment (bottom). (B) Oxygen tension measurement in the MBC. Reprinted with permission from Pathak et al. [18]. (C, left to right) Representative phase contrast light micrographs (upper panel) and wide-field fluorescence images (lower panel) obtained after fluorescent anti-CD31 mouse monoclonal antibody staining of HUVECs on tissue culture plastic (1:50 dilution) (left), on ECM gel (1:50 dilution) (center), and at the end of an MR experiment (1:10 dilution, 20-minute incubation time) (right). The control experiments revealed specific staining of HUVECs (C, left), but neither the PC-3 cells (data not shown) nor the ECM gel was stained (C, center). Despite the higher background fluorescence due to the autofluorescence of Biosilon beads, a capillary-like structure staining positively for CD31 was identified in images taken at the end of a 3-day experiment with the MBC (C, right, arrows), confirming the presence of viable HUVECs. Wide-field fluorescence and corresponding phase contrast micrographs were captured on an inverted microscope (ECLIPSE TS100) equipped with a digital camera (Coolpix 990; Nikon Corporation, Tokyo, Japan). An excitation bandwidth of 450 to 490 nm and an emission bandwidth of 500 to 550 nm were used for fluorescence imaging.