Table 3.
Cross coupling of iodoenaminones and arylboronic acids.a
| α-Iodo enaminone | Product | Substitution (R1, R2, R3) | Yield (%)b |
|---|---|---|---|
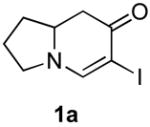
|
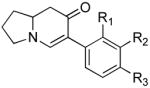
|
1b (H, H, OMe) | 70 |
| 1c (H, H, OBn) | 71 | ||
| 1d (H, NO2, H) | 57 | ||
| 1e (Cl, Cl, H) | 60 | ||
| 1f (H, H, H) | 65 | ||
| 1g (H, H, OH) | 45 | ||
| 1h (H, OMe, OMe) | 75c | ||
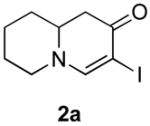
|
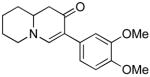
|
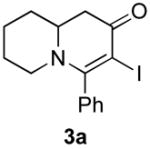
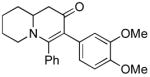
2b (as shown) | 72 |
|
|
3b (as shown) | 60 |
|
|
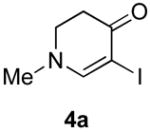
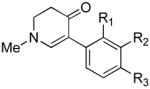
4b (H, H, OMe) | 60 |
| 4c (H, H, OBn) | 71 | ||
| 4d (H, NO2, H) | 62 | ||
| 4e (Cl, Cl, H) | 50 | ||
| 4f (H, H, H) | 55 | ||
| 4g (H, H, OH) | 36 | ||
| 4h (H, OMe, OMe) | 68 | ||
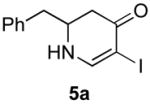
|
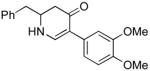
|
5b (as shown) | 70 |
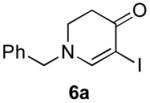
|
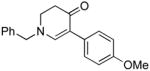
|
6b (as shown) | 69 |
