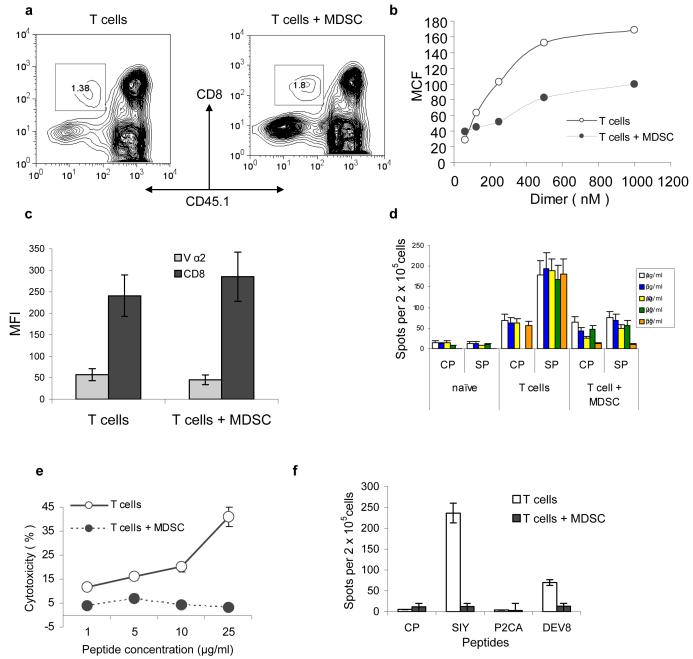
Figure 1. MSDC disrupt binding of pMHC to CD8+ T cells.
(a) OT-1 T cells (CD45.1−) were transferred to naïve congenic (CD45.1+) recipients. Adoptive transfer of MDSC and immunization were performed as described in Methods. LN cells were collected 10 days later and were gated for donor's CD45.1− CD8+ T cells. T cells alone – no MDSC transfer was performed. Typical example of 8 performed experiments is shown.
(b) CD45.1− CD8+ OT-1 T cells were incubated with various amount of PE-conjugated MHC-Ig (1.20 × 10−6 to 6.25 × 10−8 M). Binding was determined by flow cytometry. MCF-mean channel fluorescence. The level of fluorescence of non-specific dimer (SIYRYYGL-Kb-Ig) was subtracted from the values of fluorescence obtained with specific dimer (SIINFEKL- Kb-Ig). T – T cells from control mice T+MDSC- T cells from mice which received OT-1-T cells + MDSC. Three experiments with similar results were performed.
(c) The expression of CD8 and Vα2 TCR from OT-1 mice was evaluated in antigen specific CD45.1− CD8+ T cells from control and tolerized mice. The mean fluorescence ± st. dev. from three different experiments are shown.
(d) Adoptive transfer of OT-1 cells, MDSC and immunization were performed as described above. Ten days later LN cells were restimulated in triplicates for 48 h with specific or control peptides at indicated concentrations. IFN-γ producing cells was scored in ELISPOT assay and calculated per 2×105 LN cells. Three experiments with the same results were performed. CP – re-stimulation with control peptide, SP- re-stimulation with specific peptide (SIINFEKL). Naïve – no adoptive transfer of OT-1 T cells was performed; T-cells – adoptive transfer of OT-1 T cells but no MDSC; T cells + MDSC − adoptive transfer of both OT-1 T cells and MDSC.
(e) Experimental design was as described above. Additional immunization was performed on day 8. Splenocytes were isolated on day 10 and tested in a standard 6 h 51Cr-release CTL assay against the target EL-4 cells loaded with specific or control peptides at indicated concentrations. Cells were incubated in duplicates at 25:1 effector : target ratio. The levels of non-specific cytotoxicity (EL-4 target cells loaded with control peptide) were subtracted. Background cytotoxicity was less than 10%. Two experiments with similar results were performed.
(f) 2C T cells were transferred to naïve recipients followed by transfer of MDSC and immunization as described above and in Fig. S1. The following peptides were used for immunization: SIYRYYGL (SIY), LSPFPFDL (P2CA), and EQYKFYSV (DEV8). CP – control peptide (SIINFEKL). LN cells were collected 10 days later and were re-stimulated with corresponding peptides in an IFN-γ ELISPOT assay. Each group included three mice.