Abstract
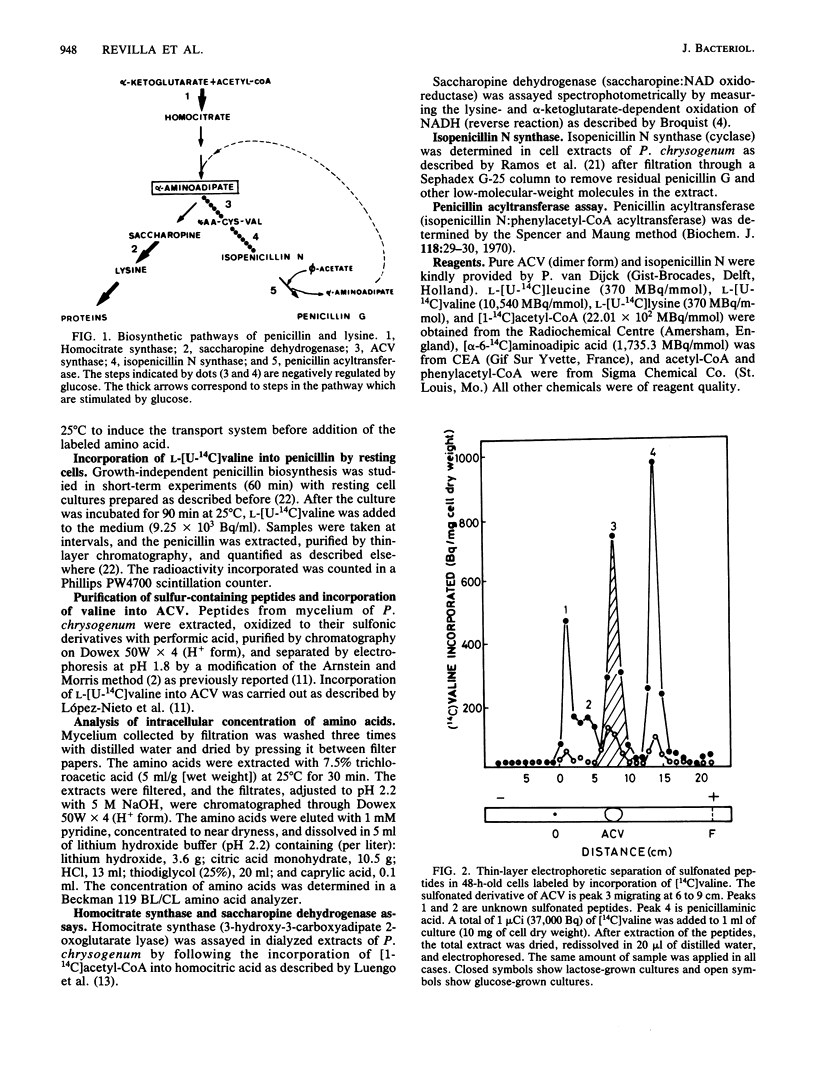
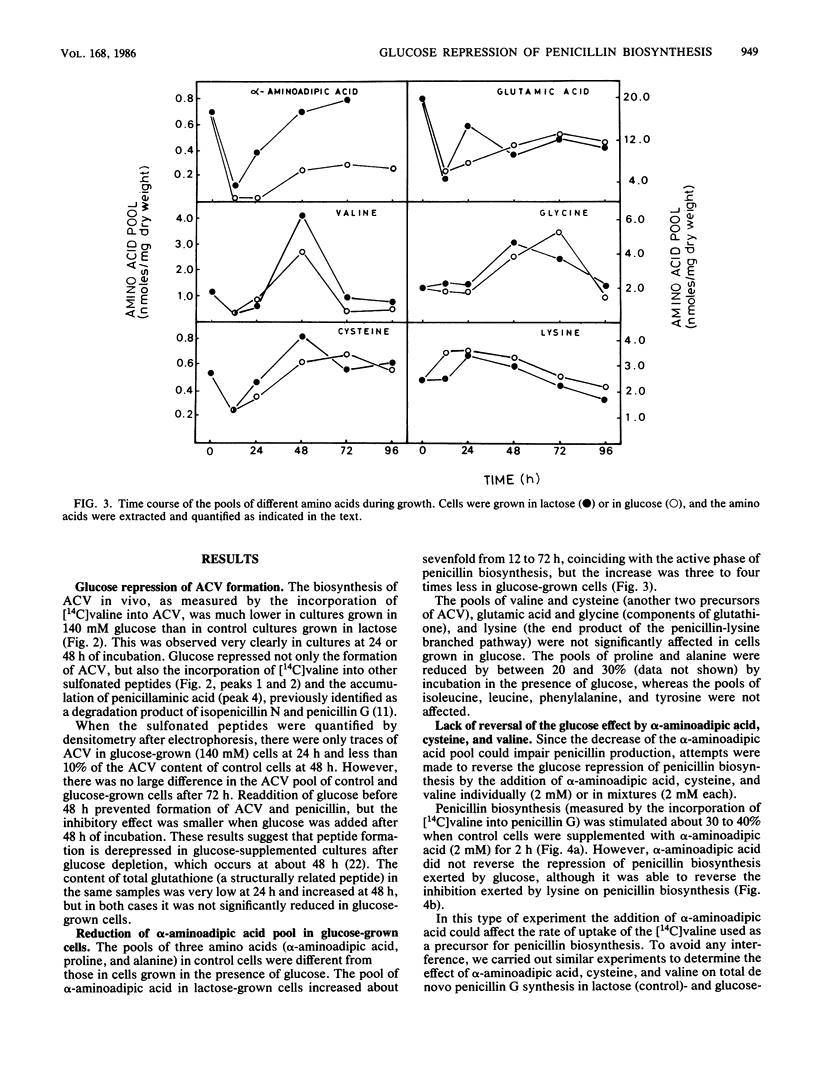
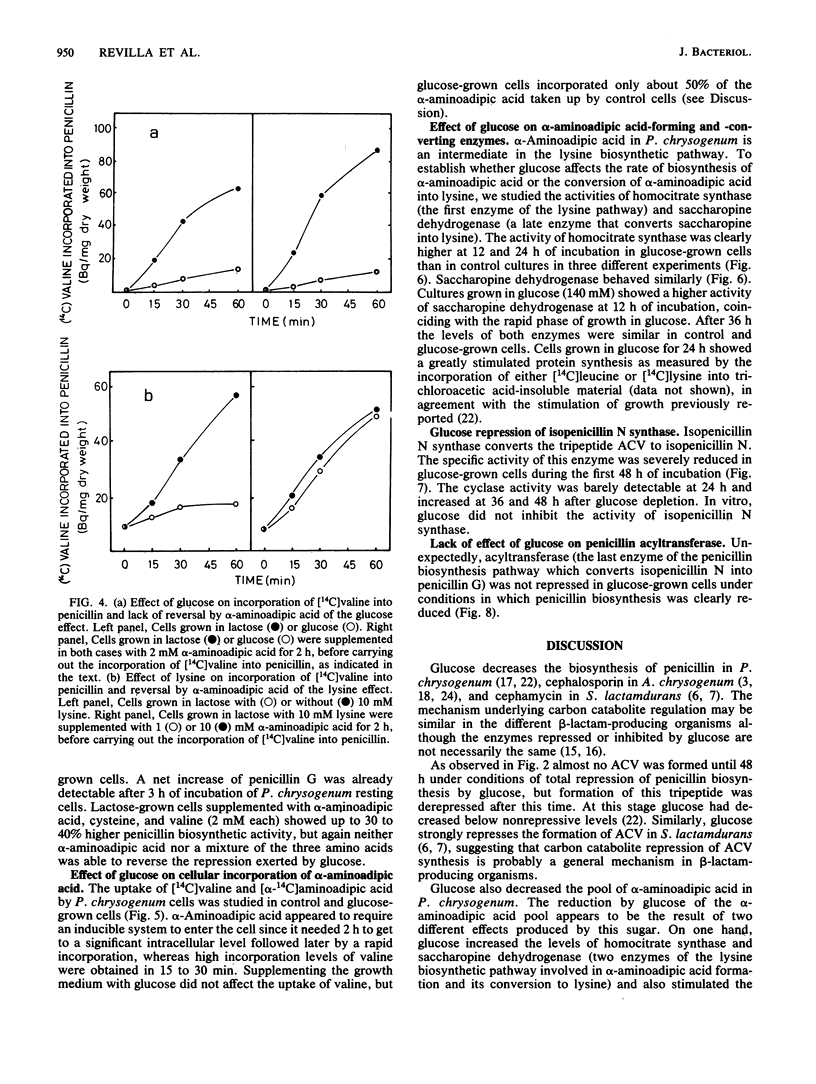
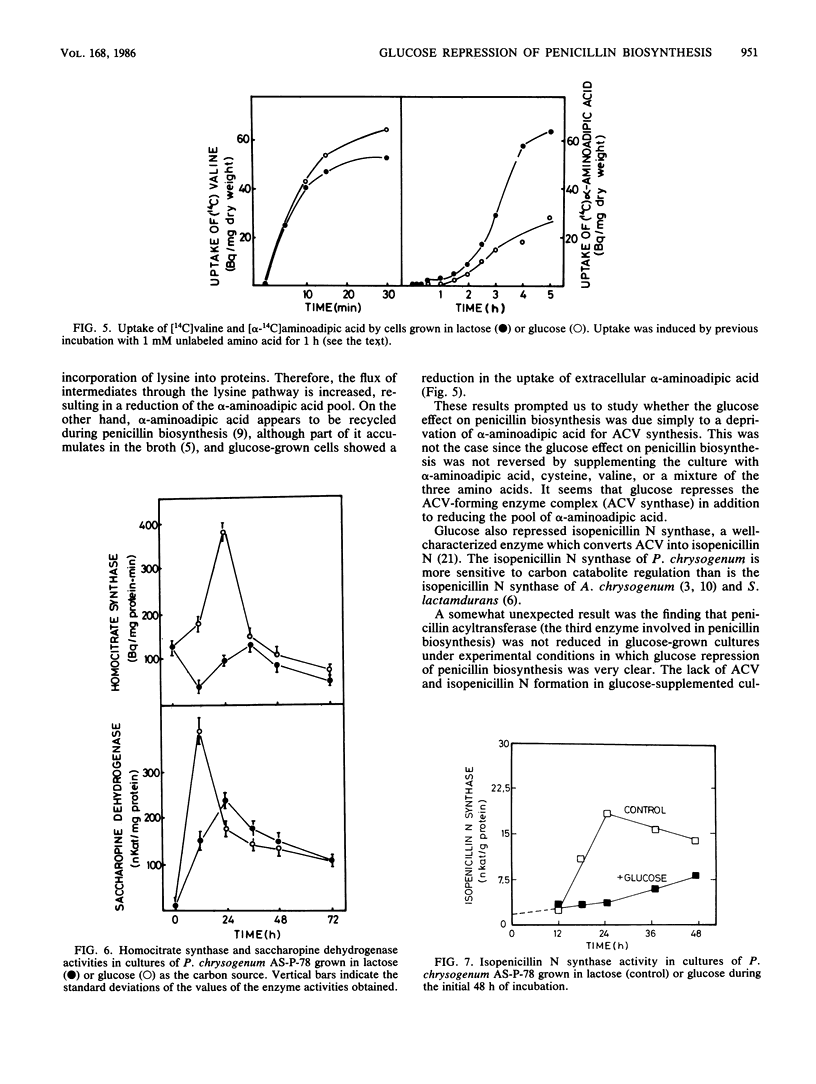
The content of alpha-aminoadipyl-cysteinyl-valine, the first intermediate of the penicillin biosynthetic pathway, decreased when Penicillium chrysogenum was grown in a high concentration of glucose. Glucose repressed the incorporation of [14C]valine into alpha-aminoadipyl-cysteinyl-[14C]valine in vivo. The pool of alpha-aminoadipic acid increased sevenfold in control (lactose-grown) penicillin-producing cultures, coinciding with the phase of rapid penicillin biosynthesis, but this increase was very small in glucose-grown cultures. Glucose stimulated homocitrate synthase and saccharopine dehydrogenase activities in vivo and increased the incorporation of lysine into proteins. These results suggest that glucose stimulates the flux through the lysine biosynthetic pathway, thus preventing alpha-aminoadipic acid accumulation. The repression of alpha-aminoadipyl-cysteinyl-valine synthesis by glucose was not reversed by the addition of alpha-aminoadipic acid, cysteine, or valine. Glucose also repressed isopenicillin N synthase, which converts alpha-aminoadipyl-cysteinyl-valine into isopenicillin N, but did not affect penicillin acyltransferase, the last enzyme of the penicillin biosynthetic pathway.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNSTEIN H. R., MORRIS D. The structure of a peptide, containing alpha-aminoadipic acid, cystine and valine, present in the mycelium of Penicillium chrysogenum. Biochem J. 1960 Aug;76:357–361. doi: 10.1042/bj0760357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonowitz Y., Demain A. L. Carbon catabolite regulation of cephalosporin production in Streptomyces clavuligerus. Antimicrob Agents Chemother. 1978 Aug;14(2):159–164. doi: 10.1128/aac.14.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundidge S. P., Gaeta F. C., Hook D. J., Sapino C., Jr, Elander R. P., Morin R. B. Association of 6-oxo-piperidine-2-carboxylic acid with penicillin V. Production on Penicillium chrysogenum fermentations. J Antibiot (Tokyo) 1980 Nov;33(11):1348–1351. doi: 10.7164/antibiotics.33.1348. [DOI] [PubMed] [Google Scholar]
- Cortés J., Liras P., Castro J. M., Martín J. F. Glucose regulation of cephamycin biosynthesis in Streptomyces lactamdurans is exerted on the formation of alpha-aminoadipyl-cysteinyl-valine and deacetoxycephalosporin C synthase. J Gen Microbiol. 1986 Jul;132(7):1805–1814. doi: 10.1099/00221287-132-7-1805. [DOI] [PubMed] [Google Scholar]
- Fawcett P. A., Usher J. J., Huddleston J. A., Bleaney R. C., Nisbet J. J., Abraham E. P. Synthesis of delta-(alpha-aminoadipyl)cysteinylvaline and its role in penicillin biosynthesis. Biochem J. 1976 Sep 1;157(3):651–660. doi: 10.1042/bj1570651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich C. G., Demain A. L. Uptake and metabolism of alpha-aminoadipic acid by Penicillium chrysogenum Wis 54-1255. Arch Microbiol. 1978 Oct 4;119(1):43–47. doi: 10.1007/BF00407926. [DOI] [PubMed] [Google Scholar]
- Luengo J. M., Revilla G., López M. J., Villanueva J. R., Martín J. F. Inhibition and repression of homocitrate synthase by lysine in Penicillium chrysogenum. J Bacteriol. 1980 Dec;144(3):869–876. doi: 10.1128/jb.144.3.869-876.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luengo J. M., Revilla G., Villanueva J. R., Martín J. F. Lysine regulation of penicillin biosynthesis in low-producing and industrial strains of Penicillium chrysogenum. J Gen Microbiol. 1979 Nov;115(1):207–211. doi: 10.1099/00221287-115-1-207. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D. M., Martín J. F. Carbon catabolite regulation of the conversion of penicillin N into cephalosporin C. J Antibiot (Tokyo) 1983 Jun;36(6):700–708. doi: 10.7164/antibiotics.36.700. [DOI] [PubMed] [Google Scholar]
- Martin J. F., Demain A. L. Control of antibiotic biosynthesis. Microbiol Rev. 1980 Jun;44(2):230–251. doi: 10.1128/mr.44.2.230-251.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang C. P., Chakravarti B., Adlington R. M., Ting H. H., White R. L., Jayatilake G. S., Baldwin J. E., Abraham E. P. Purification of isopenicillin N synthetase. Biochem J. 1984 Sep 15;222(3):789–795. doi: 10.1042/bj2220789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruess D. L., Johnson M. J. Penicillin acyltransferase in Penicillium chrysogenum. J Bacteriol. 1967 Nov;94(5):1502–1508. doi: 10.1128/jb.94.5.1502-1508.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos F. R., López-Nieto M. J., Martín J. F. Isopenicillin N synthetase of Penicillium chrysogenum, an enzyme that converts delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine to isopenicillin N. Antimicrob Agents Chemother. 1985 Mar;27(3):380–387. doi: 10.1128/aac.27.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revilla G., López-Nieto M. J., Luengo J. M., Martín J. F. Carbon catabolite repression of penicillin biosynthesis by Penicillium chrysogenum. J Antibiot (Tokyo) 1984 Jul;37(7):781–789. doi: 10.7164/antibiotics.37.781. [DOI] [PubMed] [Google Scholar]


